Nanotechnology in Dentistry: An Update
Abstract
A review of application of nanotechnology within current dental practice and related research is outlined and with reference to the scale of objects encompassed within nanotechnology. While main application areas relate to nanoparticle composition within restorative composite materials, reference is also made to applications within preventive dentistry. Attention is also given to safety factors in all aspects of development and utilisation of nanotechnology.
Introduction
Nanotechnology is an expression which has several interpretations and connotations. The potential of assembling atoms at the smallest scale of fabrication of matter was first popularly described by Richard Feynman around 1959 in his lecture on the theme of ‘There’s plenty of room at the bottom’. A keynote of this line of thinking was the potential to assemble physical structures at the scale of the atomic level. Eric Drexler 1 in 1986 proposed the possibility of construction by suitable ‘engines’ at the atomic scale. So far this level of massively parallel fabrication at the atomic scale has not been realised.
It was not, however, until the development of devices such as the scanning tunnelling microscope around 1981 that the physical manipulation of atoms could be demonstrated in the laboratory where the physical positions of atoms could be detected by means of electron current conducted from a very small tip onto the sampled surface. The historical provenance of Richard Feynman who had been awarded the Nobel prize for Physics in 1965 was then actively linked with the dramatic opportunities demonstrated by such an imaging technology. This allowed the firm establishment of the branch of science known as nanotechnology. Figure 1 indicates the groundbreaking image of Xenon atoms arranged on Nickel which first demonstrated in 1990 that atoms could be manipulated with precision at the atomic scale. Figure 2 indicates the various stages of creation of the ‘circular corral’ of iron atoms on copper.
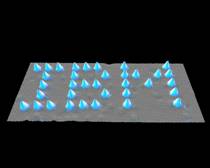
Figure 1: IBM logo constructed from Xenon atoms on Nickel atoms using scanning tunnelling microscope at IBM’s Almaden Research Centre (originally created by IBM Corporation).
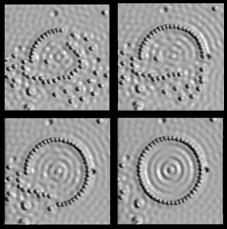
Figure 2: The Making of the Circular Corral: Iron atoms on Copper atoms imaged using a scanning tunnelling microscope at IBM’s Almaden Research Center. The diameter of the corral is 14.2 nm and comprises 48 atoms (originally created by IBM Corporation).
Today nanotechnology is generally accepted as the branch of science that deals with things between 0.1 nm (10-10 metres) than 100 nanometers (10-7 metres) and includes the manipulation of individual molecules. Individual atoms have sizes in the range 0.1 nm to 0.3 nm. The size of molecules is essentially determined by the summed length of component bonds, so that a relatively simple molecule of 1. 2,4,6-trinitro-toluene (TNT) has a total length of around 1 nm. A useful resource to identify range of size of objects from nanotechnology upwards is provided by the National Nanotechnology Initiative (http://www.nano.gov/html/facts/The_scale_of_things.html).
A level of anticipation of the potential of nanotechnology to contribute significantly to routine dental practice has previously been indicated 2. Since this was published there have been developments across a wide range of fields within nanotechnology. There has progressively emerged, however from 2000 onwards, a greater awareness of the potential hazards of nanotechnology and which is reflected in a general expression of caution with regard to human and environmental exposure for processes of manufacture, research and incorporation of nanomaterials into industrial and consumer products.
Basic Types of Nano Structures
Structures based on carbon feature prominently in research within nanotechnology. Carbon nanotubes can be either single walled or multi-walled where each ‘wall’ is fabricated from a one atom thick of carbon. Carbon nanotubes can be several millimetres long and as small as 0.7 nm. Carbon nanotubes have great potential to produce ultra strong but light weight structures. Fullerenes represent structures where carbon atoms form symmetrical structures involving from 28 atoms to over 100 atoms. In terms of shape they resemble that of a football. The best known form is that of C60 which incorporates 60 carbon atoms. While these structures have been actively investigated for a range of clinical applications, none would appear to be current within routine dentistry, though there are generally identified applications in cancer therapy 3. Graphene can be thought of as flat sheets of single atomic thickness carbon atoms which are packed densely in a honeycomb crystal lattice.
Dendrimers represent a new means of creating three dimensional macromolecules where there is a tree like branching of symmetry in the arrangement of atoms within the molecule. Applications of dendrimers are mainly related to targeted drug delivery. Nanoshell entities are nanoparticles which comprise a dielectric core (such as silica) and which are covered in a thin metallic shell – typically gold. Nanoshells have been developed as a cancer therapy 4 because after their introduction into tumour cells they can be made to absorb infra red laser radiation to result in temperature elevation within the cell leading to its death. Polymeric micelles are molecular ‘vehicles’ used to deliver specific pharmacological agents which in themselves may be poorly absorbed within the body and are widely used in a range of anti-cancer formulations 5.
Applications within Dentistry
Nanoparticle Composites
Conventional methods of manufacturing of filler materials have used materials such as quartz, melt glasses or ceramics with mechanical techniques to pulverise to powder, though this tends not to reduce particle sizes below 100 nm. ‘Hybrid’ conventional filler particles are typically 1000 nm in size and with ‘micro hybrids’ having a slightly smaller average diameter. Processes have been identified for the manufacture of sub 100 nm nanofiller materials using radically different techniques which bring new characteristics to the performance of restorative materials containing them. The incorporation of larger filler particles in the ‘hybrid’ type filler materials provides for greater mechanical strength compared to that of a material containing only nanofiller materials. The surface profile of a hybrid type composite will tend to demonstrate reduced gloss with mechanical wear – where the wear surface becomes pitted with gaps from missing filler particles and scatters light more strongly. The much smaller size of nanofiller composite will develop an exposed surface which scatters light to a smaller extend and which provides for an improved gloss performance. Even for nanofiller particles of diameter 10 nm, however, these can be estimated to contain of the order of a million individual atoms.
The compound Filtek Supreme XT compound utilises discrete non agglomerated aggregated nanofiller particles in range 20 to 75 nm and also nanoclusters which are loosely bound agglomerates of nano sized particles. The agglomerates allow for a high level of filler loading which provides high bond strength. Figure 3 outlines the characteristics of the wear surface of a ‘Hybrid’ composite and of Filtek Supreme restorative material where additional mechanical strength is provided by the nanoclusters but the small size of component nanofiller material gives improved gloss performance. Mitra and Holmes 6 compare properties of mechanical strength, wear resistance and gloss retention of Filtek Supreme Translucent and Filtek Supreme Standard with five other restorative materials (Filtek 250; TPN Spectrum; Point4; EsthetX; and Filtek A110). Filtek Supreme Standard was found to have best wear resistance and best gloss retention up to 300 brush cycles and mechanical strength characteristics among the top three of the group tested.
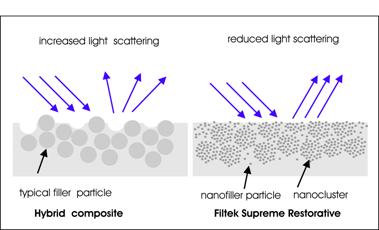
Figure 3: Comparison of surface characteristics of ‘Hybrid’ composite and Filtek Supreme restorative showing how surface roughness influences degree of light scattering and associated gloss performance (not to scale).
A specific study has been undertaken by Ernst et al 7 in which comparison was made of the effectiveness of nanofiller resin composite Filtek Supreme (3M ESPE) with conventional fine hybrid resin composite Tetric Ceram (Ivoclar Vivadent) in stress-bearing posterior cavities. A study group of 50 patients received at least one of both types of restorative material. No statistically significant differences were found between the two restorative materials after two years. A more detailed review of nanotechnology developments within composite materials is provided by Saunders 8.
Regenerative Endodontics
Fioretti et al 9 describe the possibility of using a nano-sized film containing alpha melanocyte stimulating hormone (alpha-MSH) to use its anti-inflammatory properties to fight inflammation in dental pulp fibroblasts. Laboratory experiments indicate the ability of growth of human pulp fibroblasts with such nanolayers This is a component of regenerative endodontics which advocates a broad range of techniques for restoration of tooth viability in which diseased or necrotic pulp tissues are removed and replaced with healthy pulp tissue to revitalize teeth. A broader review of regenerative endodontics is provided by Murray 10.
Caries Inhibition in Fillings
The addition of compounds such as dicalcium phosphate anhydrous, or DCPA to the resin mix in a composite filing is conventionally undertaken to provide a steady release of calcium and phosphate ions to inhibit caries development and strengthen tooth metabolism. The inclusion of such materials, however, can structurally weaken the filling as a whole. Investigators 11,12 have described how nano sized particles of DCPA and similar agents are more effective as ion release agents and that the reduced levels of DCPA lead generally to higher bond strength.
Tooth Surface Conditioning
The potential advantageous use of silica nanoparticles to polish tooth surfaces has been identified by Gaikwad and Sokolov 13 where areas of teeth polished with silica nanoparticles demonstrated reduced adherence of cariogenic bacteria such as Streptococcus mutans.
It is widely accepted that the reduction of bacterial adherence and biofilm formation on tooth surfaces has the potential to reduce the incidence of caries and periodontitis. One group 14 has identified that coating tooth surfaces with a commercially available organic/inorganic nano-composite coating significantly reduced the surface loading of both enamel and titanium samples which were exposed over a 24 hour period within a volunteer group. It is identified that further work in this area is required to determine the effectiveness and intrinsic safety of such nano-composite coatings over clinically relevant time periods. A recent review of the role of nanotechnology in preventive dentistry is outlined by Hannig 15.
Tooth Surface Characteristics: Nanoindentation
The technique of nanoindentation 16 allows characterisation of material surfaces by determining force/displacement curve as force is applied to an indentation probe on the surface of the test material. He and Swain 17 describe the use of a nanoindenter to characterise the response of tooth enamel where nanoindentor tip diameters as small as 5 microns are used and where the penetration depth can be determined with high sub nm accuracy. It was identified that the surface of enamel produces an inelastic response which is associated with a very small protein rich component which exists between the hydroxyapatite nanocrystals and also within the protective structure surrounding the enamel rods.
Safety Concerns
Developing Safety Frameworks
The extensive report from Health and Safety Executive in 2004 18 made a range of key statements regarding risks and safety assessments of nanoparticles which highlighted major shortcomings within the then current procedures. The risk associated with nanotechnology is identified as two distinct types. The current focus is on the effect of uptake of nanomaterials within biological structures and environmental systems. The other and more perplexing risk is the potential for nano structures in the form of assemblers/dissasemblers at the atomic/molecular level to modify the atomic/molecular structure of tissue. It is this scenario of the potential of nanostructures to reduce living tissue to ‘grey goo’ which was initially described by Drexler 1 . Currently, nanotechnology does not have the potential to create such assemblers/dissasemblers and such a scenario is generally accepted as exceedingly unlikely.
A more recent report 19 confirms anxiety about the range of knowledge of occupational health and safety aspects related to synthetic nanoparticles. There appears to be an indication that the smaller particles of nanoparticles may demonstrate more toxic effects than material present as particles of larger dimensions, but same material. The report identifies a specific concern with carbon nanotubes which appears to show toxicity in animal studies 20 similar to that of asbestos and is causing significant concern in the international scientific community.
Titanium Dioxide
Titanium dioxide is a commonly used material within a broad range of cosmetic and household products, including toothpaste. Investigators 21 have demonstrated that at a body loading of 5 g/Kg in mice, nanoparticles of typical size 25 nm and 80 nm could be transported to the liver, spleen, kidneys, and lung tissues after uptake by the gastrointestinal tract. In addition, at the specific dose levels administered, tissue damage was observed in the liver and kidney. Specific evidence of genetic damage in mice with exposure of Titanium dioxide nanoparticles has been identified 22. It has been estimated 23 that the fraction of production of Titanium dioxide in the USA as nanoparticles is progressively increasing from a low base level but will be almost totally in the nanoparticle formulation by 2026.
Nano Silver
Particles of silver at the nano scale or ‘nanosilver’ are being incorporated into a range of consumer products such as soap, shampoo and toothpaste to exploit their germ killing properties. A review by Tolemat et al 24 has identified that most production processes produce spherical silver nanoparticles with diameters of less than 20 nm. It is considered important, however, to consider separately the relative safety of colloid silver particles and inonic silver particles. Chi et al. 25 have indicated that while the genotoxicity of nanoAg is weak, obvious genotoxicity can be demonstrated with the agent cetlpyridine bromide (CPB) which is a compound found in some mouthwashes, toothpastes and lozenges. The implication of the study is that nanoAg and CPB are present in combination there is the potential for genotoxic activity. Techniques for evaluation of exposure of nanoparticles from consumer products including toothpaste are described by Benn et al. 26.
It is important, however, to clearly understand the scope of research which may prove some formulations a potential hazard while others are shown to be low risk. In the context of carbon nanotubes, for example, while longer length carbon nanotubes are considered to present a potential hazard, short length tubes may present a significantly lower level of hazard.
Conclusion
Utilisation of nanotechnology within dentistry is largely in evidence through the use of smaller filler particle sizes within restorative composites, though research has also highlighted a range of options to implement nanotechnology within a range of preventive measures. It is relevant to comment that clinical papers referencing applications of nanotechnology infrequently reference safety issues which appear to be taken more seriously by the wider scientific nanotechnology community.
References
1 K. Eric Drexler Engines of Creation : The Coming Era of Nanotechnology, Anchor Books, 1986
2 Ure D, Harris J. Nanotechnology in dentistry: reduction to practice. Dent Update 2003; 30:10-5.
3 Partha R and Conyers JL. Biomedical applications of functionalized fullerene-based nanomaterials, Int J Nanomedicine 2009; 4: 261–275.
4 Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2:282-289.
5 Benny O, Fainaru O, Adini et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol. 2008;26:799-807.
6 Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials.J Am Dent Assoc. 2003;134:1382-1390.
7 Ernst CP, Brandenbusch M, Meyer G, Canbek K, Gottschalk F, Willershausen B. Two-year clinical performance of a nanofiller vs a fine-particle hybrid resin composite, Clin Oral Investig. 2006;10:119-125.
8 Saunders SA. Current practicality of nanotechnology in dentistry. Part 1. Focus on nanocomposite restoratives and biomimetics, Clinical Cosmetic and Investigational Dentistry, 2009
9 Fioretti F, Mendoza-Palomares C, Helms M et al. Nanostructured assemblies for dental application. ACS Nano. 2010;22;4:3277-3287.
10 Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377-390.
11 Xu HH, Weir MD, Sun L, Ngai S, Takagi S, Chow LC. Effect of filler level and particle size on dental caries-inhibiting Ca-PO(4) composite. J Mater Sci Mater Med. 2009;20:1771-1779.
12 Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC et al. Strong nanocomposites with Ca, PO(4), and F release for caries inhibition. J Dent Res. 2010;89;19-28.
13 Gaikwad RM, Sokolov I. Silica nanoparticles to polish tooth surfaces for caries prevention. J Dent Res. 2008;87:980-983.
14 Hannig, M.Kriener, L, Hoth-Hannig W, Becker-Willinger C, Schmidt H, Influence of Nanocomposite Surface Coating on Biofilm Formation In Situ,Journal of Nanoscience and Nanotechnology, 2007;7: 4642-4648.
15 Hannig M and Hannig C, Nanomaterials in preventive dentistry, Nature Nanotechnology, 2010;5:565-569.
16 Fisher-Cripps Laboratories Pty. Ltd,The IBIS Handbook of Nanoindentation, Australia, 2009: http://www.ibisonline.com.au/downloads/downloads/IBIS_HandbookOfNanoindentationBook.pdf
17 He LH, Swain MV. Energy absorption characterization of human enamel using nanoindentation. J Biomed Mater Res A. 2007;81:484-492.
18 Health and Safety Executive, Nanoparticles: (2004) An occupational hygiene review, prepared by the Institute of Occupational Medicine.
19 IRSST publications,Engineered Nanoparticles: Current Knowledge about Occupational Health and Safety Risks and Prevention Measures, Report R-656 – Second Edition, Quebec, 2010
20 Craig A, Poland CA, Duffin R et al., Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study, Nature Nanotechnology.2008;3:423 – 428.
21 Wang J, Zhou G, Chen C et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176-185.
22 Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;15:69(22):8784-8789.
23 Robichaud CO, Uyar AE, Darby MR, Zucker L, Wiesner MR, Estimates of Upper Bounds and Trends in Nano-TiO2 Production As a Basis for Exposure Assessment, Environ. Sci. Technol. 2009;43:4227–4233.
24 Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ. 2010;408:999-1006.
25 Chi Z, Liu R, Zhao L, Qin P, Pan X, Sun F et al. A new strategy to probe the genotoxicity of silver nanoparticles combined with cetylpyridine bromide. Spectrochim Acta A Mol Biomol Spectrosc. 2009;72:577-581.
26 Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. J Environ Qual. 2010;39:1875-1882.