General Principles of Toxicology using Caenorhabditis elegans as a model
1.1 General Principles of Toxicology
An aphorism attributed to Paracelsus (1493–1541), Toxicology is a multidisciplinary science dealing with the study of the adverse effects and hazardous nature of chemical and physical agents which ultimately produce a qualitative and quantitative measurable reaction in living organisms exposed to specific conditions. Experimental toxicology also investigates the occurrence, incidence and mechanism of action of risk factors affecting the organism under experimental scrutiny (Williams et al., 2000).
According to Williams et al. 2000, the four main conceptual aspects of toxicology are:
- Exposure – contact between toxicant and organism, likely to cause damage to the latter;
- Dose – quantity of a toxicant administered to an organism at one or more time intervals;
- Absorbed dose – actual intake of a toxicant that is absorbed into the organism;
- Effective target dose – quantity of toxicant reaching the target organ with adverse effects.
Beyond this, the mechanism of toxicity is defined by the various biological responses and interactions determining what the toxicant triggers within the organism. There is a key difference between a toxicant – which denotes any chemical compound provoking adverse effects at a sufficient concentration in a living organism – and a toxin – which denotes any toxicant produced by an organism (animal, plant or microbial) as part of its offensive or defensive strategy. These aspects are integrated into any standard toxicity test (Williams et al., 2000), governed by five essential types of selection: (a) of a test organism; (b) of a measurable quantitative and/or qualitative response; (c) of a suitable exposure period; (d) of a suitable observation period; and (e) of a series of doses to test.
1.2 Caenorhabditis elegans as an experimental model
1.2.1 Relevance of Caenorhabditis elegans as an animal model in toxicology
This opportunistic free-living nematode colonises disturbed soils and, presents many practical advantages for toxicological testing. These include its unrivalled characterisation at a genetic, molecular and developmental level; its simple culture requirements for growth at 15°C to 25°C on Petri plates or in liquid culture feeding on Escherichia coli bacteria; its rapid lifecycle with 0.5d linked to short generation time of 3.5d at 25°C; doubling time of half a day associated with a short generation time of 3.5d (zygote to zygote); its reproduction by either self- or cross-fertilisation; its small size; and its high reproductive rate producing a large number of offspring (200–300 per hermaphrodite adult) which allows easy maintenance of large numbers on Petri dishes or in aerated liquid cultures. Moreover, frozen liquid nitrogen stocks can be maintained indefinitely (≈ at -70°C) without genetic drift because C. elegans (Figure 1.2.1.1) inhabits the film of water surrounding soil particles, so toxicity testing is possible in both aquatic and soil systems. However, it is relatively resistant to many single stressors, although more sensitive to mixtures (Mutwakil et al., 1997), non-ionic surfactants in combination with heavy metals facilitate metal entry into tissues (Dennis et al., 1997) and increase overall toxicity. Reproductive deficits are also seen in C. elegans exposed to metals at different developmental stages (Guo et al., 2009). Taken together, these factors make this small metazoan organism an ideal genetic model system for toxicology, as well as for exploring the neurobiology and genetics of development in higher organisms (Burglin et al., 1998). C. elegans has a small genome and the complete sequence (100 million bp) was published over 10 years ago (C. elegans sequencing consortium, 1998 [Science]). Since then, a variety of post-genomic technologies, such as RNA interference (Kenneth et al., 2003), have been applied to elucidate the functions of its ≈20,000 genes – an unexpectedly high number for such an apparently simple organism.
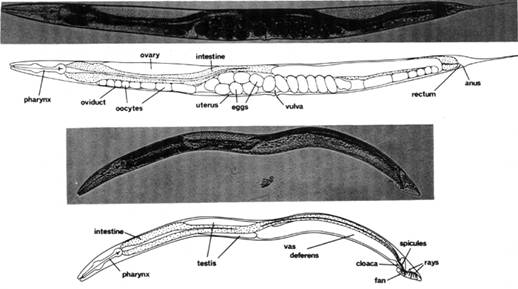
Figure 1.2.1.1 – Caenorhabditis elegans anatomy. Photomicrographs showing major anatomical features of the C. elegans adult hermaphrodite (top) and male (bottom). Shown are lateral views under bright-field illumination. Bar, 20 μm.
Source: Riddle et al., 1997.
Last but not least, the small size and simple culture requirements of C. elegans greatly reduce the costs and technical demands of toxicity assays using this species. Collectively, these features allow C. elegans to be used for collecting any toxicological data on the biological effects of the many pollutants produced by human activities.
1.2.2 Developmental biology of C. elegans
Development progresses through four larval stages, L1 to L4 (Figure 1.2.2.1), each stage lasting between 7h and 11h, at 25°C. The majority (> 99%) of the individuals are self-fertilising XX hermaphrodites, but rare XO males (≈0.2%) arise by X chromosome non-disjunction at meiosis. A single hermaphrodite can produce 300–350 progeny, while hermaphrodites mated with males can yield more than 1000 progeny. Mating through male animals is essential when genetic crosses are to be performed, but self-fertilisation allows maintenance of recessive mutations in homozygous populations. An alternative developmental strategy (dauer larval stage) may be followed by L1 larvae facing adverse environmental conditions, enabling the organism to survive with enhanced stress resistance and a greatly increased metabolic rate for several months, compared with a normal lifespan of ≈20 days (Burglin et al., 1998).
Embryogenesis generates about 550 cells and postembryonic cell divisions roughly double the cell number (a hermaphrodite adult has 959 somatic nuclei while the adult male has 1031 somatic nuclei), with the final adult animals reaching about 1.2 mm in length. Differential interference contrast microscopy is commonly utilised for assessing development due to the animals’ transparency, but there is not much alteration in overall form during development from L1 to adult (Burglin et al., 1998). The simple nematode structure displays a wide variety of cell types including muscles, pharynx, intestine, hypodermis (responsible for the cuticle and the basement membrane) and 302 neurons grouped into 118 morphologically different types. The complexity of the nervous system enables C. elegans to show a large variety of behaviours and responses, many of which have been analysed genetically (Burglin et al., 1998).
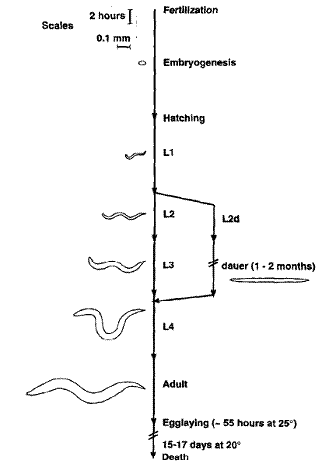
Figure 1.2.2.1 – Schematic diagrams of the larval stages (L1–L4) of the lifecycle of Caenorhabditis elegans. The time line is approximately to scale, except where indicated by breaks; a scale is given in the upper left. The right-side diversion represents the alternative developmental dauer pathway.
Source: (Burglin et al., 1998).
1.2.3 Structure and function of the C. elegans cuticle and basement membranes
The cuticle (Figure 1.2.3.1) is a multilayered structural exoskeleton characteristic of nematodes, composed of proteins (about 90%), lipids and carbohydrates (around 10%). The cuticle is responsible for maintaining morphology, protection, mobility and interactions with the outer environment (transport of nutrients and detection of stimuli). By contrast, basement membranes cover most of the internal organs and their main role is to function as physical barriers separating them from the pseudocoelomic space (Burglin et al., 1998; Page & Johnstone, 2007). Three major groups of protein define the structure of the impervious cuticle: collagens (major connective tissue in animals), specialised insoluble proteins known as cuticlins, and surface-associated molecules (glycoproteins and lipids) (Page & Johnstone, 2007). There are clear stage-specific ultrastructural changes as well as protein expression changes (stage-specific collagen, etc.) during development from L1 larvae through to the adult.
The dauer larvae cuticle differs ultrastructurally from that of the adults. These dauer larvae are highly modified L3 larvae which do not feed because their intestine is sealed off at the mouth and anus. Their cuticle is strengthened and resistant to treatment with harsh chemicals; also, their metabolic activity is reduced and depends on internal energy stores: They are resistant to environmental stresses such as heat, cold, oxidative stress (from reactive oxygen species (ROS)) and desiccation; however, their chemosensory system is functional and they are capable of rapid movement. Stress resistance, cellular maintenance and detoxification pathways are upregulated in dauer larvae: in addition to the increased activity of anti-ROS defence systems (described above), dauer larvae also show increased transcriptional activity of other cellular defence systems, such as heat-shock protein (HSP) genes.
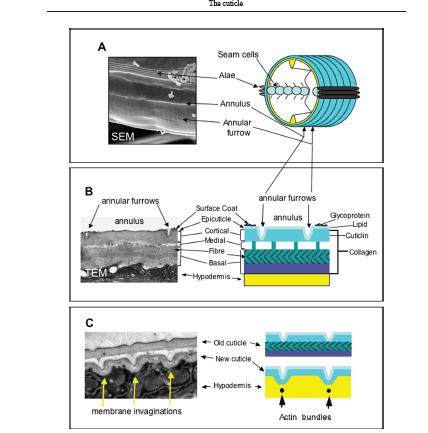
Figure 1.2.3.1 – The organisation and structure of the C. elegans cuticle.
Panel A – scanning electron micrograph (SEM) of the surface of a wild-type adult-stage animal and an accompanying schematic representation.
Panel B – transmission electron micrograph (TEM) depicting a longitudinal cross-section of the adult cuticle highlighting the distinct structural layers and their composition.
Panel C – synthesis of a new cuticle and associated detachment of the old cuticle.
Source: Page and Johnstone, 2007.
1.2.4 Model-to-human extrapolation
Bearing in mind regulatory requirements and the necessity of predicting human responses to environmental and man-made toxicants (since direct human toxicity data is usually lacking), there is a crucial need for qualitative and quantitative response similarities between the model organism and human beings. The fact that different species widely possess differing response systems, even under the same test conditions (dosage, period of exposure, etc.) requires a paradigm when establishing appropriate animal models. This is especially true for the invertebrates, such as C. elegans, that are far removed from humans in evolutionary terms. Toxicologists frequently design appropriate experimental tests based on preliminary knowledge of what the human response is likely to be.
Nevertheless, selection of the best (or least inadequate) animal model is complicated by the numerous and often vast differences between species, in terms of their physiology, biochemistry and anatomy aspects (Williams et al., 2000). Such differences can and do produce significant variations and divergent responses. When comparing different test species, for this reason, two different invertebrates (C. elegans and D. melanogaster) will be employed in an attempt to model responses of a conserved network of stress genes – although only the former (C. elegans) is used in the work described here.
The extrapolation of animal responses to humans should be viewed as a very difficult, uncertain and complex process. Different test species may differ in terms of: (a) basal metabolic rates; (b) anatomy and organ structure; (c) physiology and cellular biochemistry, (d) cellular toxicodynamics, and (e) bioactivation/detoxification of chemicals and their metabolic intermediates (Williams et al., 2000).
1.2.5 Methods for genetic expression pattern analysis in C. elegans
Various methods to study the expression patterns of protein selected genes have been established: (a) immunolocalisation using specific antibodies to determine expression patterns at cellular and subcellular level; (b) in situ hybridisation using DNA or RNA probes to identify where particular genes are being transcribed; (c) transgenic technology using reporter genes – one such widely used reporter is the jellyfish Green Fluorescent Protein (GFP) (Chalfie et al., 1996); molecular cloning of the GFP gene to heterologous systems (prokaryotic or eukaryotic recipients) allows its subsequent expression to be monitored by fluorescence methods. The past 15 years have established recombinant GFP as a valuable reporter molecule for in vivo visualisation of gene expression. In view of the fact that recombinant GFP requires no additional substrates or cofactors for fluorescence, it can be localised using fluorescence microscopy or quantified (as a measure of expression) using fluorometry. The fluorescent signal from recombinant GFP represents a relative value that can be quantified by comparison to a known recombinant GFP standard curve.
1.2.6 The corollary of two models (C. elegans and D. melanogaster)
Caenorhabditis elegans has become a very popular model system for genetic and molecular research, thus it was chosen as a model system or the first metazoan Genome Sequencing Project (C. elegans Sequencing Consortium, 1998). So far, the functions of most of these genes remains mysterious but it is accepted that 53% show homology to genes in other organisms (nearly 10303 genes) (Kim et al., 2008). The older genetic model organism Drosophila melanogaster has emerged as a powerful model system for a number of human neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, and other diseases such as diabetes, cancer and recently human heart conditions (Wolf et al., 2006). The ability to generate thousands of P-element insertions throughout its genome confers a unique advantage for screening and rapid mapping of candidate genes; this is not currently possible for other model organisms such as the mouse. For obvious reasons, genotoxicity studies are gaining more attention from researchers worldwide, where both nematode (Sobkowiak and Lesicki, 2009) and fruit fly (Chroust et al., 2007) systems provide optimal indicators of mutagenic and carcinogenic activity in response to various chemical compounds.
Molecular phylogenetic analyses initially supported the idea that the phylum Nematodas branched off at an early stage of animal evolution, prior to the split between protostomes (including Drosophila) and deuterostomes (humans), but after the triploblastic acoelomate body plan (flatworms) (Burglin et al., 1998). Recent sequencing experiments propose that nematodes are instead the basal branch of a clade of sloughing animals, called the ecdysozoa (Aguinaldo et al., 1997). Nevertheless, analysis of developmental control genes, such as the Hox gene clusters suggests a very early branching of nematodes from other animals; C. elegans contains only four authentic Hox genes, compared to nine in Drosophila and over 30 in vertebrates (Burglin et al., 1998). It is therefore clear that the difference between these two models is important; the classical analysis suggests that genes common in both Drosophila and C. elegans should also be present in humans (Burglin et al., 1998), whereas the more recent model allows the possibility that any such common gene could derive from an ecdysozoan ancestor and, thus, unfortunately need not be present in humans.
1.2.7 A gene expression map for Caenorhabditis elegans
The gene expression map for C. elegans (19282 genes) is a three-dimensional expression map displaying correlations of gene expression profiles, based on information from DNA microarrays, genetic mutants and gene expression studies for different growth conditions and developmental stages. This gene expression interface provides a powerful tool for the assessment of known co-regulated groups of genes and can also be utilised to reveal previously unknown genetic functions (Kim et al., 2001).
1.3 Predictive Modelling
1.3.1 Classic prediction models
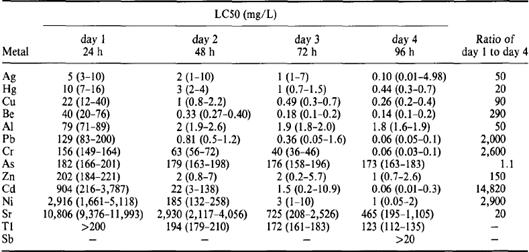
Environmental pollution has increased dramatically due to human interference along with growing industrialisation producing a broad spectrum of pollutants. The toxicological impact of these pollutants on organisms and their ecological niches requires testing, but the number of hazardous substances involved highlights an impracticality that calls for models which can generate accurate predictions. The toxicity of metals playing a major role in water pollution, such as Ag, Hg, Cu, Be, Al, Pb, Cr, As, Tl, Zn, Cd, Ni, Sr and Sb (derived from industrial waste discharges or natural occurrence of concentrated sources of metals in soils), has been tested in C. elegans lethality assays (Williams & Dusenbery, 1990) producing a large-scale database of toxicological comparison (Table 1.3.1.1). Tatara et al. (1997) then derived Quantitative Structure Activity Relationships (QSAR) from these data – a technique commonly used to predict the toxicity of organic compounds by relating the chemical and physical characteristics of a compound to its observed toxicity, thereby accounting for variation in toxicity for a related group of chemicals.
Tatara et al. (1997) believe that it is possible to make general predictions about relative metal toxicity from ion characteristics. This study also showed that identification and quantification of all bioactive metal species does not necessarily improve prediction of relative metal toxicity with ion characteristics.
Table 1.3.1.1 – Aquatic LC50 values during four days of exposure. The T1 day 1 LC50 is a lower limit due to solubility limitations. The SB value is the highest concentration obtainable due to solubility limitations.
Source: Williams and Dusenbery, 1990.
Nowadays, two models are frequently used to predict mixture toxicity from single compound dose response data: (a) concentration addition (CA), known as dose addition or Loewe additivity, based on the assumption that the mixture components act on the same biochemical pathway and same molecular target, the only difference between mixture components being their relative potencies (developed by Loewe and Muischnek in 1926); or (b) independent action (IA) (also known as Bliss independence, response addition, or effect multiplication), based on the assumption that the distribution of the sensitivities of individuals to the toxicants is statistically independent (Martin et al., 2009). Both these models postulate no interactions occurring between the component chemicals within the sample mixture, and their mathematical meaning is explored in Martin et al. 2009. The result is that there is a direct cause-effect relationship. The accuracy of mixture toxicity predictions using the CA and IA models has been assessed in a number of studies (Altenburger et al., 1996; Martin et al., 2009), but the inability of these models to consistently and accurately predict mixture toxicity has led to proposals for additional corrections in the equations.
I have so far briefly discussed the fundamental concepts of toxicology with a view towards developing models for predicting mixture toxicity. Due to the enormous range of toxicants available nowadays, their ubiquitous presence, diverse nature and wide distribution, there is much to be done in this field. The following will briefly explore the most relevant properties of the investigated toxicants, namely, heavy metals and pesticides.
1.3.2 Properties and effects of heavy metals
Oehme (1978) describes heavy metals as persistent, slowly eliminated environmental agents that act as inducers of specific toxic effects. Williams et al (2000) describe heavy metals as “[varying] greatly in their physical and chemical properties, and therefore, in their potential for absorption and toxicity”. Some metals are essential for good health, but at higher concentrations can be toxic. Some can remain in the body for significant periods of time, stored in specific tissues, to be slowly released and excreted via urine and faeces.
A number of toxic heavy metals (Table 1.3.2.1), listed by regulatory environmental bodies (EPA, ACGIH, EA and OSHA) were tested in this research against C. elegans stress-response reporters.
Table 1.3.2.1 – Properties and known effects of tested heavy metals. All information extracted from Williams et al. (2000), unless stated otherwise.
| Compound | Nature | Toxicity | Effects on Humans | RfD and US EPA Category |
Arsenite | Inorganic compound containing arsenic (a toxic metalloid used in a number of herbicides and insecticides). | Highly variable depending on the exposed subject, the form of arsenic (As+3 vs. As+5), as well as route, rate and duration of exposure. | The main concern is increased risk of lung cancer, although respiratory irritation, nausea, and skin effects also may occur. | 0.3 µg/Kg; Group A for its known human carcinogenic actions. |
Cadmium | Pervasive and persistent environmental contaminant ranked in the Top 10 on the Comprehensive Environmental Response, and Liability Act Hazardous Substances Priority list. | Displays carcinogenic behaviour , and is known to displace zinc from various proteins, leading to cellular lesions. | Oral exposure to high concentrations of cadmium causes severe irritation to the gastrointestinal epithelium, nausea, vomiting, abdominal pain and diarrhoea, and agonising bone mass disorders. | 0.5 µg/kg from water sources, in comparison to 0.1 µg/kg daily from food sources. Class B1 carcinogen (probable human carcinogen), by inhalation only. |
Copper | Reddish metal, widely distributed in nature. Essential trace element that serves as a cofactor in many critical biological processes such as respiration, iron transport, and oxidative stress protection. | Copper ions are toxic to cells because they can adopt distinct redox states (reversibly donating and receiving electrons). High doses result in increasing toxicity causing devastating and irreparable damage to proteins, lipids and DNA | Copper deficiency causes characteristic disease symptoms. However, copper excess can be toxic and therefore its concentration in the cells needs careful balancing to preserve homeostasis. | 40 (µg/kg-day) Cuprous chloride is classified as toxicity Category I [Danger] for primary eye irritation and primary dermal irritation. |
Chromium | Can occur as three major valencies: hexavalent chromium (+6) and elemental chromium (0) are typically generated by industrial processes, whereas trivalent chromium (+3) occurs naturally in the environment. | Chromium (+3) does not readily cross cell membranes and is relatively inactive in vivo. However, intracellular chromium (+7) can react slowly with both nucleic acids and proteins and can be genotoxic. | Chromium (+6) is irritating and short-term high-level exposure can result in numerous adverse effects in the area exposed (ulcers and nasal mucosa and gastrointestinal tract, adverse effects on kidney and liver). Very corrosive and can severely burn the skin. | For chromium (+3) is 1.0 mg/kg and for chromium (+6) is 5 µg/kg. Chromium (+6) is classified as a Class A (confirmed human carcinogen) by the inhalation route of exposure only with chromium (+3) not classified as a carcinogen by any route of exposure. |
Iron | The most abundant trace mineral in the body. Essential but potentially toxic element in most biological systems. Required for electron transport and utilisation of oxygen. Can act on enzymes to catalyse free radical reactions. | Iron overload or serum transferrin deficiency are important dysregulatory conditions. Excess iron not bound to transferrin leads to iron overload in parenchyma. | High levels have been shown to cause lesions in cells and tissues, hepatocellular necrosis, hereditary diseases and carcinoma. By contrast, iron deficiency leads to pathological states such as anaemia, growth retardation, and immune system dysfunctions. | The EPA has not calculated an RfD for iron, but the Tolerable Upper Intake level for >19 years old, determined by the US Food and Nutrition Board Institute of Medicine is 45 mg/day (Goldhaber, 2003). |
Mercury | Found in the environment in many forms: the metallic or elemental form, as inorganic compounds or organic mercury compounds. | Its toxicity depends on the specificity: alkyl mercury compounds are extremely toxic in comparison to the inorganic mercury compounds. | Inorganic mercury tends to be filtered and reabsorbed in the kidneys. Organic mercury targets the brain and, to a smaller extent, the kidneys. | 0.3 µg/kg/d (inorganic) Mercury is not classified as a carcinogen by any route of exposure. |
Zinc | Essential trace element in the diet, occurs in the environment primarily in the +2 oxidation state. | The number of genes coding for proteins with zinc-binding domains is conservatively estimated at ~3–10% of the human genome. | Ingested zinc salts are corrosive and irritating to the gastrointestinal tract. Inorganic zinc is relatively nontoxic by oral exposure. | 300 µg/kg Zinc is classified in Group D (not classifiable with regard to human carcinogenicity). |
Bibliography
The bibliography has followed the Harvard System of Referencing.
Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. (2004). “Pesticides and Oxidative Stress: A Review”. Med Sci Monit,10 (6), RA141-147.
Aguinaldo, A. M. A.; Turbeville, J. M.; Linford, L. S.; Rivera, M. C.; Garey, J. R.; Raff, R. A.; Lake, J. A. (1997). “Evidence for a clade of nematodes, arthropods and other moulting animals”. Nature, 387, 489-493.
Altenburger, R.; Boedeker, W. and Grimme, L. H. (1996). “Regulations for combined effects of pollutants: Consequences from risk assessment in aquatic toxicology”. Food and Chemical Toxicology, 34, 1155-1157.
An, J. H. and Blackwell, T. K. (2003). “SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response”. Genes and Development, 17, 1882-1893.
Anokye-Danso, F.; Anyanful, A.; Sakube, Y. and Kagawa, H. (2008). “Transcription factors GATA/ELT-2 and Forkhead/HNF-3/PHA-4 regulate the tropomyosin gene expression in the pharynx and intestine of Caenorhabditis elegans. Journal of Molecular Biology, 379, 201-211.
Bolter, C. J. and Chefurka, W. (1990). “Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes”. Animal physiology and biochemistry, 278 (1), 65-72.
Bowerman, B.; Eaton, B. A. and Priess, J. R. (1992). “skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo”. Cell, 68 (6), 1061-1075.
Bryden, J. and Noble, J. (2006). “Computational modelling, explicit mathematical treatments, and scientific explanation”. School of Computing, University of Leeds.
Burglin, T. R.; Lobos, E. and Blaxter, M. L. (1998). “Caenorhabditis elegans as a model for parasitic nematodes. International Journal of Parasitology, 28, 395-411.
Calafato, S.; Swain, S.; Hughes, S.; Kille, P. and Sturzenbaum, S. R. (2008). “Knock down of Caenorhabditis elegans cutc-1 exacerbates the sensitivity towards high levels of copper”. Toxicological Sciences, 106 (2), 384-391.
Carter, M. E. and Brunet, A. (2007). “FOXO Transcription Factors”. Current Biology, 17 (4), R114.
Chalfie, M. and Prasher, D., “Uses of green-fluorescence protein”. United States Patent. (1996).
Cha’On, U.; Valmas, N.; Collins, Patrick; Reilly, P. E. B.; Hammock, B. D. and Ebert, P. R. (2007). “Disruption of iron homeostasis increases phosphine toxicity in Caenorhabditis elegans”. Toxicological Sciences. 96 (1), 194-201.
Cheeseman, K. H. and Slater, T. F. (1993). “An introduction to free radical biochemistry”. British Medical Bulletin 49, 481-493.
Chroust, K.; Pavlova, M.; Prokop, Z.; Mendel, J.; Bozkova, K.; Kubat, Z.; Zajickova, V.; Damborsky, J. (2007). “Quantitative structure-activity relationships for toxicity and genotoxicity of halogenated aliphatic compounds: Wing spot test of Drosophila melanogaster”. Chemosphere, 67, 152-159.