An intervention to determine the benefit of larval-source management for on-going control programmes in Kampala, Uganda.
INTRODUCTION
Malaria is still one of the leading causes of death in sub-Saharan Africa but there have been several advances in its control (WHO 2013).
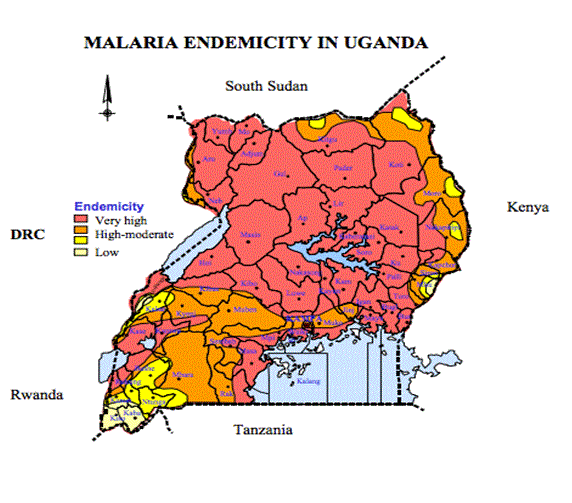
Figure 1: Malaria endemicity in Uganda.
(PMI, Uganda 2013)
The figure above shows the malaria endemicity in Uganda, where 90 per cent of the population is at risk of malaria. Ninety-five per cent of the country shows high endemicity, while the remaining five per cent of the county consists of epidemic-prone transmission areas. These are in the highlands of the south and west, the eastern border with Kenya, and the north-eastern border with South Sudan. In the northern areas of Uganda, there is a high rate of infected mosquitoes (PMI, Uganda 2013). In sub-Saharan Africa, the incidence of malaria is growing rapidly and this may have a significant impact on the epidemiology of malaria (Robert et al., 2003). Although, generally, malaria transmission is lower in urban areas than in rural areas in Africa, this is not the case in Uganda. From Figure 1, it can be seen that there is high transmission in the north. In terms of eliminating the vectors, the parasite prevalence is important and a study by Thompson et al. (1997) showed that parasite prevalence may be heterogeneous within densely populated areas, clustering near mosquito breeding sites.
In 2009, a survey conducted by the Uganda Malaria Indicator Survey (UMIS) revealed that Plasmodium falciparum is the leading cause of malaria, being responsible for 99 per cent of cases while Plasmodium malariae was reported in 0.2 per cent of cases. Cases from Plasmodium ovale and Plasmodium vivax are very rare (UMIS, 2011).
The main vectors found in Uganda are Anopheles funestus, found at higher altitudes during the dry season, and Anopheles gambiae s.l. From Figure 1 above, it is clear that there is very high endemicity in the entire population in Uganda (World Malaria Report, 2013)
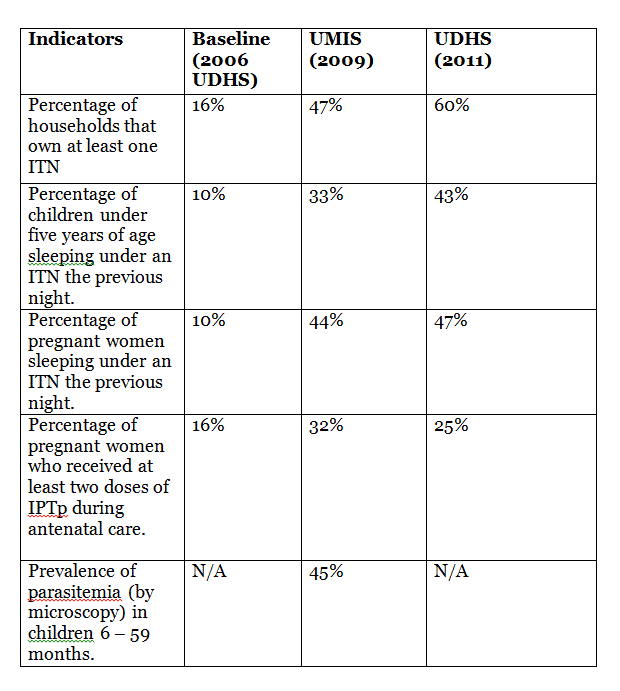
Table 1: The current situation in Kampala, Uganda: coverage and impact data from the Uganda Demographic and Health Survey (UDHS 2011) and the 2009 Uganda Malaria Indicator Survey (UMIS).
In Kampala, malaria is presently the leading cause of morbidity and absenteeism in schools and workplaces, and 60 per cent of the health budget is spent on its control (Uganda Ministry of Health, unpublished data). At present the major foci of malaria control in Kampala include case management, use of LLINs, presumptive treatment of malaria in pregnant women and environmental management (EM). EM, through a process of social mobilisation and community participation, is being encouraged by the Ministry of Health and includes filling in small water collections, clearing bushes around homes and closing windows early in the evening (World Malaria Report, 2013).
However, the purpose of LSM intervention is to establish whether this form of intervention supports malaria control and has an impact on the transmission of malaria, leading to a reduction in prevalence. Two areas in Kampala will be assessed in this intervention.
JUSTIFICATION
Some of the on-going interventions in controlling malaria in Kampala are case management, LLINs and IRS (Okia et al., 2013). This research compares LLINs with LSM. LLINs that are impregnated with insecticide are important because they act as a physical barrier protecting the sleeping individual from the vector. Increased use of LLINs will eventually reduce the density and survival of mosquitoes and reduce the frequency of their blood sucking (Gimnig et al., 2003). There is widespread resistance in Uganda due to the target site (kdr) and metabolic mechanisms. Therefore monitoring insecticide resistance is essential for vector control (Verhaeghen et al., 2010).
However, since LLINs are being distributed for free by the Government, it is important to promote and educate people of the benefits of sleeping underneath a net and how they are to be used. Wide-scale improvement in LLIN usage would eventually protect community members, including the vulnerable who do not own nets (Maxwell et al., 2002). However some studies have shown that moderate use of LLINs could actually increase the risk of malaria for unprotected people by causing the mosquitoes to divert from those using nets to those without. (Hii et al., 2001) (N’Guessan, Corbel, Akogbeto & Rowland, 2007) (Hargreaves et al., 2000).
The aim of the intervention reported here was to target the aquatic areas in one site using LSM to determine whether this would be beneficial as a control measure. Larvicides were introduced in 2012 (World Malaria report 2013) and the Ministry of Health introduced them in Uganda, using Temephos (Abate) to target breeding sites in northern Uganda. The NMPC also used larvicides to target man-made and natural breeding sites in central Kampala before extending their use to other districts (Miti, 2012).
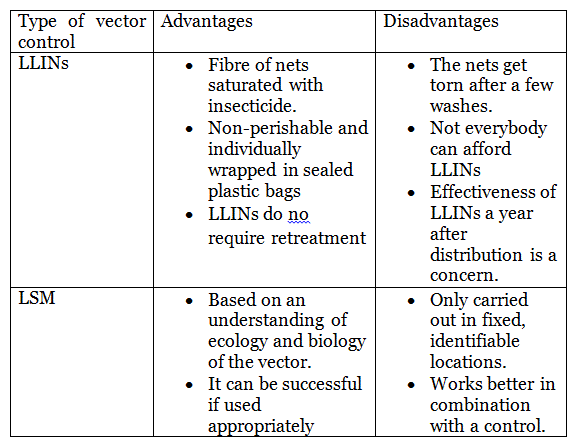
Table 2: The advantages and disadvantages of the use of LLINs and larva-source management.
The major importance of LSM is that it targets the aquatic stages of the vector while LLINs target the adult stage of the mosquito. Both control programmes will reduce the abundance of mosquitoes and the transmission of malaria. LSM will target burrow pits, ponds, pools, roadside puddles, canals and riverbed pools. This intervention will last for a year with initial parasitological and entomological data collected as a baseline while working with local health authorities. This intervention will improve the relationship among the local health authorities working in vector and environmental control.
DETAILS OF THE INTERVENTION
This intervention will focus on two areas in Kampala where there were many defined breeding grounds. It is a pilot study to investigate the effects of adding a larvicide in one area to reduce malaria transmission. In the other area, no larvicides will be used and only LLINs will be used to combat malaria transmission.
First, the current malaria prevalence will be assessed by doing a cross sectional study to identify the areas at high risk of malaria. Local transmission will be confirmed by the following:
- A survey of net ownership and usage to be undertaken by going from house to house interviewing people, taking blood samples and using a rapid diagnostic test with those running temperatures or who are taking anti-malarial medication.
- Vectors to be sampled (biting rate, species, endophagic, endophilic). This will be repeated at both sites.
- A survey of the breeding sites in both locations to be undertaken to sample the larva.
- Data to be collected from the health authorities on the on-going use of larvicides on both sites, if being used.
- Clinical data to be collected from the hospitals or health-care centres concerning treatment and diagnosis of the populations at both sites.
Once the baseline cross-sectional survey has been done in areas where there is confirmed malaria transmission, the community health workers or larvicide technicians will map out which potential habitats need larvicide treatment. They will also take into account what the land is used for and ownership will be assessed.
To carry out this intervention we will need the following sets of people, who will all be trained:
- Clinicians
- Laboratory technicians
- Larvicide technicians
- Trainee Entomologist
- Community health worker
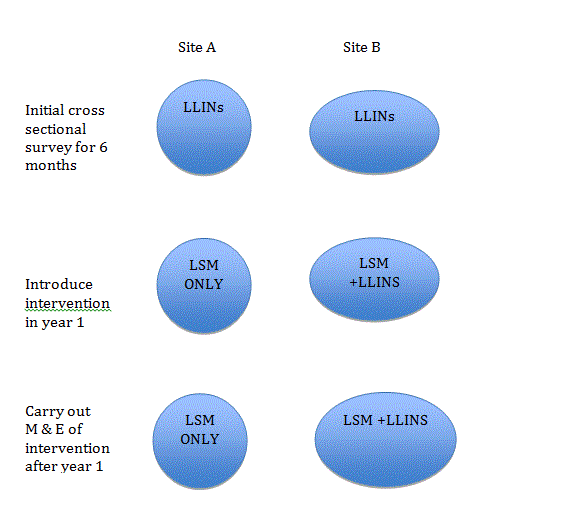
Figure 3: Study Design of intervention in two sites in Kampala.
Educational campaign for the importance of LLINs.
- Schools to introduce the importance of LLINs into the school curriculum.
- Community health workers to go from house to house with questionnaires on ownership and to promote the use of nets already owned.
- Television and radio commercials as well as printed leaflets.
- Distribution of printed material and insecticides.
Larval Source Management.
This will include the type of LSM to use: in this case, the application of biological insecticides to bodies of water. The soil bacterium Bacillus thuringiensis especially Bacillus israelensis is used to interfere with the larval digestive system. Bti (VectoBac) is at a rate of 2.5 to 10 Ibs per acre, depending on the organic properties of the water. The insecticide needs to be applied weekly in all habitats. It will kill mosquitoes for 30 days or longer when applied to standing water. It is also important to understand the larval ecology and status of vector resistance (Lindsay et al., 2004). The ratio of human population density to larval habitat density will be taken into account.
Separate teams from those responsible for larval surveillance should conduct the process. Staff employed in the larval survey using dippers should be trained and, for example, each larval control officer could be given responsibility for treating one plot. Supervisors should provide the mosquito-control officers with a timetable for plots to visit each day and the entire area should be searched for standing water on the sites. All larval habitats should be targeted and treated if wet at the time of visit, irrespective of the presence or absence of larvae, and visited every week (Lindsay et al., 2004).
Some studies (Fillinger & Lindsay, 2006; Fillinger, Ndenga, Githeko, & Lindsay, 2009) have shown the importance of combing a vector control with larvicide as this can result in an overall reduction in malaria, adult mosquitoes and aquatic stages by more than 90 per cent.
We will exclude private hospitals because there might be a difference in case management. We will also exclude places that have already been targeted with larvicide.
MONITORING AND EVALUATION
This intervention will determine the impact and benefit of LSM in one location after comparing the findings with the location not treated with larvicide and only LLINs. After one year another cross sectional survey will be carried out to determine the reduction of malaria prevalence from the site treated with LSM and LLINs and the site with LLINs only.
(A) Data collection
- The use of nets in a community, which takes into account the number of households with LLINs (coverage), the number of nets used each night (adherence) and the number of nets still saturated with insecticide (treatment).
- Larva collection of mosquitoes from both sites by using dippers and information from the larval surveillance team.
- Adult mosquito collection by sampling the vectors on both sites with the use of light traps or the catch-and-release method.
- The benefit of educational awareness on the use of LLINs by going to houses and schools with a questionnaire.
- Hospital data to compare the site treated with larvicide with the site not treated with larvicide treatment.
(B) Measurement indicators for the expected outcome
- Overall proportion of prevalence of clinical malaria and RDTs positives before introducing LSM to one only.
- Overall proportion of incidence of clinical malaria in site without LSM and promotion of the use of LLINs.
Ten trained workers will collect this information every week and clinical data from the hospital will be collected every month before the next intervention. M & E is important as it can improve the programme, identify future resources needed and collect information for future policy and advocacy. Monitoring will be carried out internally while evaluation will be carried out both externally and internally. The internal evaluators will have knowledge about the context and operations. With the correct and additional use of LSM, it is expected that there will be a reduction in malaria prevalence treated with bti. If this pilot study is successful, we can extend this to the other areas in Kampala (Lindsay et al., 2004).
TIMELINE AND RESOURCES
There will be a budget of USD 30,000 per year with weekly monitoring and evaluation, according to the baseline method described above. This study will last for one year. This decision is based on other studies that have been done, especially the study carried out in Kampala by Lindsay et al., (2004) who looked at two cities in Uganda.
Staff training: All staff will be trained in mosquito collection, microscopic diagnoses and the use of RDTs. They will also be trained to do larval surveys and the application of bti. Training will last for four weeks at take place at the local hospital.
Education campaign: The cost of teaching the local people the value of LLINs and promoting their use will be borne by the community health workers. Transport will be provided for people going from house to house. Funding will be provided for the printing of posters and materials. LLINs will be given to those who do not have them.
Larvicide: The costs of LSM must be carefully considered. The cost per person (US$ 0.94 and US$ 2.50) per year of those on the area identified for the study was determined by the ratio of human population density to larval habitat. The costs include the formula and the equipment required.
Funding: This will be from the Ministry of Health Uganda, the London School of Hygiene and Tropical Medicine and other bodies in Uganda.
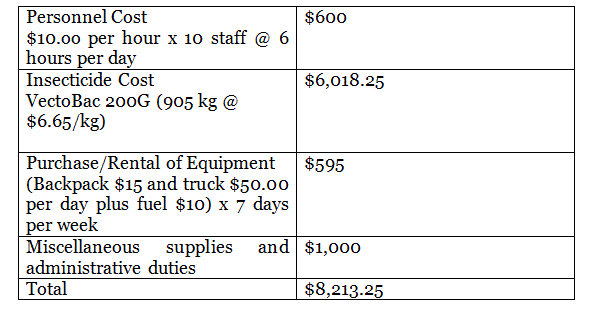
Table 3: Larviciding budget for one site.
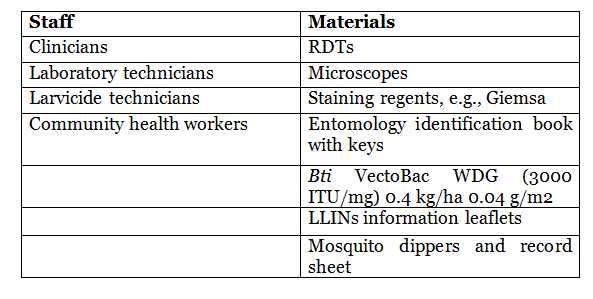
Table 4: Staff and materials
ETHICS
Before the study commences, ethical consent will be authorised from the Ministry of Health in Uganda and from Uganda National Council for Science and Technology.
- All participants will sign an informed consent document.
- Permission will be obtained from parents and guardians.
- Community consent and cooperation will be obtained.
- Ethical clearance will be received from home institution
REFERENCES
Fillinger, U. & Lindsay, S. W. (2006). Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health, 11(11), 1629-1642. doi: 10.1111/j.1365-3156.2006.01733.x
Fillinger, U., Ndenga, B., Githeko, A., & Lindsay, S. W. (2009). Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ, 87(9), 655-665.
Gimnig, J. E., Vulule, J. M., Lo, T. Q., Kamau, L., Kolczak, M. S., Phillips-Howard, P. A., . . . Hawley, W. A. (2003). Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg, 68(4 Suppl), 16-22.
Hargreaves, K., Koekemoer, L. L., Brooke, B. D., Hunt, R. H., Mthembu, J., & Coetzee, M. (2000). Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol, 14(2), 181-189.
Hii, J. L., Smith, T., Vounatsou, P., Alexander, N., Mai, A., Ibam, E., & Alpers, M. P. (2001). Area effects of bednet use in a malaria-endemic area in Papua New Guinea. Trans R Soc Trop Med Hyg, 95(1), 7-13.
Innitiative, P. M. (2014). Malaria Operational Plan for Fiscal Year 2014.
Lindsay, S. W., Egwang, T.G., Kabuye, F, T. Mutambo , T & Matwale,G,K. (2004). Community-based Environmental Management Program for Malaria Control in Kampala and Jinja, Uganda Final Report (pp. 1-62).
Maxwell, C. A., Msuya, E., Sudi, M., Njunwa, K. J., Carneiro, I. A., & Curtis, C. F. (2002). Effect of community-wide use of insecticide-treated nets for 3-4 years on malarial morbidity in Tanzania. Trop Med Int Health, 7(12), 1003-1008.
Miti, J. (2012). Ministry launches larvicides for Malaria fight. Daily Monitor.
N’Guessan, R., Corbel, V., Akogbeto, M., & Rowland, M. (2007). Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis, 13(2), 199-206. doi: 10.3201/eid1302.060631
Okia, M., Ndyomugyenyi, R., Kirunda, J., Byaruhanga, A., Adibaku, S., Lwamafa, D. K., & Kironde, F. (2013). Bioefficacy of long-lasting insecticidal nets against pyrethroid-resistant populations of Anopheles gambiae s.s. from different malaria transmission zones in Uganda. Parasit Vectors, 6, 130. doi: 10.1186/1756-3305-6-130
Robert, V., Macintyre, K., Keating, J., Trape, J. F., Duchemin, J. B., Warren, M., & Beier, J. C. (2003). Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg, 68(2), 169-176.
Statistics, U. B. o. (2011). Uganda Demographic and Health Survey 2011.
Thompson, R., Begtrup, K., Cuamba, N., Dgedge,M., Mendis,C., Gamage-Mendis ,A., Enosse, S., Barreto, J., Sinden, R., Hogh, B., . (1997). The Matola Malaria Project: a temporal and spatial study of malaria transmission and disease in a suburban area of Maputo, Mozambique. Am J Trop Med Hyg 57, 550-559.
Verhaeghen, K., Bortel, W. V., Roelants, P., Okello, P. E., Talisuna, A., & Coosemans, M. (2010). Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. Am J Trop Med Hyg, 82(4), 566-573. doi: 10.4269/ajtmh.2010.08-0668
WHO. (2013). World Malaria Report (pp. 284). Geneva.