To what extent can it be argued that the acidification of soils in humid temperate regions is the product of natural soil development as opposed to land-use practice?
Introduction
Acidification of soils is caused by the leaching and otherwise removal of cations (e.g. Ca and Mg) at a rate faster than can be supplied by the weathering of the parent material. Leaching most often occurs where precipitation exceeds evapotranspiration, and includes the temperate regions of north-western Europe, where soils have been subject to leaching since the end of the last ice age (Ellis & Mellor, 1995). Natural soils facing the same conditions for an extended time reach an equilibrium pH, but a wide variety of causes can change pH. Very low pH can cause a variety of problems, such as reducing the availability of nutrients for plant growth and mobilising heavy metals such as aluminium, which can be toxic when washed into fresh water systems.
Natural causes
Pure rainwater in equilibrium with the atmosphere has a pH of 5.6. On meeting soil with a lower pH, the water is a sink of protons, as HCO3– à H2CO3. However, the effect is a relatively small increase in pH, since the concentration of bicarbonate ions is low (Rowell & Wild, 1985). When meeting soil with a pH above 5.6, rain has an acidifying effect, as carbonic acid dissociates, releasing protons. These protons exchange with the exchangeable cations such as Ca and Mg, which are then leached out of the system. In calcareous soils with pH >6.5, CaCO3 dissolves to Ca2+ and HCO3– which are also lost by leaching. The effect of rainwater as an acidifying agent is strongest in this last soil type (Rowell & Wild, 1985).
Microbial respiration causes acidification by releasing CO2 which dissolves in soil water to form carbonic acid. Plants also contain many organic acids that are released to the soil. Nitrifying bacteria living in the soil cause acidification by contributing to organic decomposition. In this process, NH4+ ions are oxidised to NO3– ions, with H+ ions as a by-product.
Plant growth itself causes acidification, as base cations taken up by the roots are exchanged for H+ ions. The type of vegetation has a great effect on the contribution to acidity. Much research has been done into the effects of different tree species and woodland types (e.g. Hornung, 1985; Ovington, 1953) since the development of forests generally causes soil acidification. The processes in a new forest that contribute to acidification are the formation of an acid litter and mor humus, throughfall and stemflow (altering the chemistry of rainwater), production of organic acids, increased base cation uptake from the soil and increased precipitation interception and evapotranspiration. Not all of these processes act under all forests, and the effects are most marked under coniferous forests. However, there is evidence that acidification of soils beneath conifers is largely reversed when the trees reach maturity. During the early years humus build-up is rapid, but later, as the canopy closes, the microclimate becomes less favourable for decomposition of litter and, thus, nitrification by bacteria (Miles, 1985). The high soil pH created by young forests also affects the soil macrofauna, particularly earthworms. Earthworms are efficient decomposers of organic material, but can’t tolerate low pH. So, the decomposition of litter under a mature forest is further hindered by the loss of earthworm populations, and pH rises. Rising pH during forest maturity is also due to changes in the ground vegetation as the forest closes in; for example, the loss of relict moorland vegetation that has a strong acidifying effect.
Natural grass and moorland vegetation have a larger range of effects, depending on the species. However, many temperate grasslands have pHs of 4–5, and heather moorlands have slightly lower pHs (Thompson & Loveland, 1985), as they have a tendency to produce acid litter and mor humus.
Parent material, local conditions and climate all affect the susceptibility of soil to acidification. The most vulnerable soils are thin and freely drained, with acidic parent material (e.g. granites) and are found in wet, cool climates. Large areas of upland Britain meet these criteria and therefore have naturally acidic soils (Billett et al. 1988).
Causes due to land use and management
Many natural causes of acidification can be exacerbated by land-use changes and practices. Afforestation, particularly of conifer woodlands, can cause acidification according to the natural processes outlined above. When trees, or any other plants, are cropped, the cations they have taken up during their life are prevented from being returned to the soil system. Thus, cations are removed from the system, and the proportion of H+ increases. Similarly, clearing dead trees and plant material prevents recycling of cations. When land is cleared of vegetation and water is lost from the binding system, the soil is aerated, and base metal cations are oxidised to mineralised forms, thereby removing them from the soil complex and increasing acidity. While the development of a forest can cause acidification, so can its removal, if replaced by a greater acidifier. For example, the clearing of indigenous hardwood forests across western Europe caused acidification, but the area had reached equilibrium. In some areas the forests have been replaced by heath vegetation, which has further increased soil acidification (Smith & Taylor, 1969). Not all forest planting causes acidification, as many species of trees do not produce such acid litter and mor humus. However, artificial forests are often planted in areas where soil quality is already poor and with species of tree that produce acidity. As with land clearance, drainage of land also leads to mineralisation via aeration, especially where there is a large quantity of sulphide minerals.
The effect of grazing grass and moorlands is less clear, as it depends on which species are palatable to the grazers! Some grasses, if heavily grazed, can improve soil pH, while others naturally produce a deep littler layer and acid mor humus. In general, grazing actually improves pH of natural grasslands.
A major source of soil acidification is the fertilisation of agricultural land. Most inorganic fertilisers are nitrogen-based, as salts. As in the natural process, plants that take up NH4+ release H+ ions in their place. Where NH4+ ions are not taken up, they are oxidised by natural nitrifying bacteria. When the resulting NO3– is in excess of plant needs, they behave as mobile ions, encouraging leaching (Ellis & Mellor, 1995).
Contributions of causes
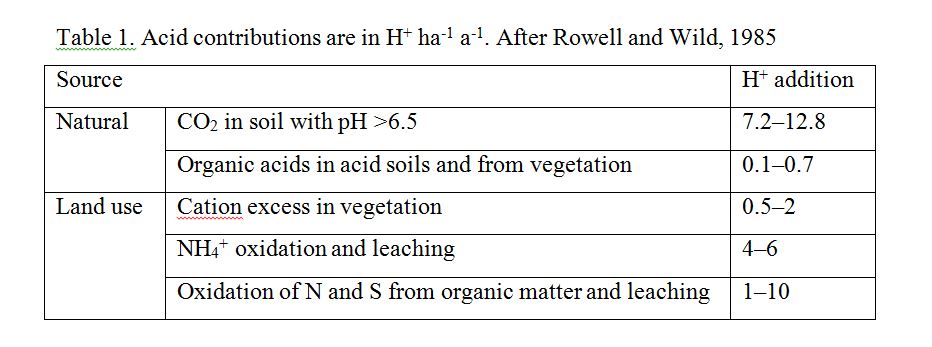
Rowell and Wild (1985) summarise the causes of acidification and estimate the contribution of several causes (Table 1). They show that CO2 addition to soils has the greatest impact, but is important primarily for carbonate soils. The second largest contribution then, is from NH3 and NH4+ oxidation in agricultural soils, and associated leaching. This should cause a massive decrease in soil pH, but in reality, the acidity is combated by farmers who neutralise the soil by liming.
The effect of tree species on soil acidity depends on both initial site conditions and silvicultural management, but there are general trends – one such trend is that conifers tend to intensify increased acidity of the upper soil to a greater extent than hardwood species (Ovington, 1953). For example, on relatively nutrient-rich soils beech does not produce acid soil conditions, but on base-poor soils, acid soils can be produced by beech forests. However, it has been shown that even conifer planting can raise pH values if the land was originally colonised by more aggressive acidifiers, such as heath and moorland. The climatic climax vegetation for much of the UK is broadleaf forest, so if moorlands preserved for grouse hunting are given over to forests, the pH will also rise. Conversely, if grazed grasslands are left to nature, they will become scrub, adding acid to the soil.
If trees are cut down completely at each harvest, the early stages of forest development begin anew, with increased acidification of soil. Monoculture of trees, both in age and species, will cause an extreme in effect. A forest that is completely cut down at each harvest and totally replanted with young trees will be uniformly acidified at the same rate. Trees of different ages, with some mature specimens, will help keep the rate of soil acidification down. Interplanting with species known to reduce acidity will also help maintain a reasonable pH level, although if the soil is extremely poor to begin with, this may not provide much aid. Therefore, afforestation can cause acidity, but the initial soil conditions, the tree species and the cropping methods adopted for the plantation may mitigate the impact. If the trees are cropped, either a ‘crop rotation’ should be employed, as it is in traditional agriculture, or the forest should be allowed to consist of a variety of tree ages and species. In the UK, most forests are coniferous, and so the contribution to acidification by trees can be presumed to be only from conifers. This is in contrast to the climatic climax vegetation across much or Britain, and the type of woodland that covered the islands before people arrived, so the acidification due to conifers can be presumed to be largely man-made. However, where conifers replace acidic moorlands, pH can actually increase. While all these changes can be considered to be man-made, the relative net change in pH of all these changes has not been considered.
Conclusions
The relative roles of natural and anthropogenic land-use factors on soil acidification are difficult to assess. The effects of changes in land use depend on the original purpose of the land. Before any human intervention , across the temperate region of the UK the land was broadleaf forest, heath and moorland. There were some coniferous forests in the northern parts. Thus, any change from the original climate equilibrium can be considered to be anthropogenic land-use change, all of which change the acidity of the soil. Generally then, the removal of broadleaf forest and its replacement by moorland, fertilised agricultural land and coniferous forests have been human contributions to soil acidification. However, where forestation has replaced moorland, and grazing has cropped acidic shrubs, the effect is the opposite – raising pH compared to the natural situation.
Disregarding the fact that planting coniferous species does not restore the natural forest in most parts of the UK, coniferous forests, once established, would reach an equilibrium pH if natural acidification processes were allowed to continue. However, where there is cultivation of new forests, there is clear evidence that the land management causes an increase in acidity compared to the natural situation. This is primarily because cations are removed by cropping, and total clearance causes forest establishment to begin again, with the accelerated rates of acidification associated with young growth.
References
Billett, M. F., FitzPatrick, E.A., and Cresser, M. S., 1988. Long-term changes in the acidity of forest soils in North-East Scotland. Soil Use and Management 4(3): 102-107
Ellis, S., and Mellor, A., 1995. Soils and Environment London: Routledge
Hornung, M., 1985. Acidification of soils by trees and forests. Soil Use and Management, 1(1): 24-28
Miles, J., 1985. The pedogenic effects of different species and vegetation types and the implications of succession. Journal of Soil Science, 36: 571-584
Ovington, J. D., 1953. Studies of the development of woodland conditions under different trees I: Soils pH. Journal of Ecology, 41: 13-34
Rowell, D. L. and Wild, A., 1985. Causes of soil acidification: a summary. Soil Use and Management, 1(1): 32-33
Smith, R. T., and Taylor, J. A., 1969. The post-glacial development of vegetation and soils in northern Cardiganshire. Transactions of the Institute of British Geographers, 48: 75-97
Thompson, T. R. E., and Loveland, P. J., 1985. The acidity of Welsh soils. Soil Use and Management, 1(1): 21-24