LPS and its signalling receptors
Lipopolysaccharide (LPS)
LPS is made up of three main regions known as the O-antigen, the core region, and lipid A (Dunn et al., 1991) (figure 1). The O-antigen consists of repeating polysaccharide subunits that are specific for each bacterium, and thus determines its identity. The core region of LPS to which the O-antigen is attached, consists of sugars such as heptose and 2,-keto-3-deoxy-octulosonic acid as well as oligosaccharide residues which are morphologically conserved among genera of Gram-negative bacteria. The lipid A component to which the core region is attached, is the most conserved region, and consists of phosphorylated glucosamine disaccharide residues containing several fatty acids. This hydrophobic component is the moiety to which most of the biological/pro-inflammatory effects of LPS are attributed, as early studies showed that synthetic lipid A is able to elicit endotoxic and biological activity (Takada et al., 1989).
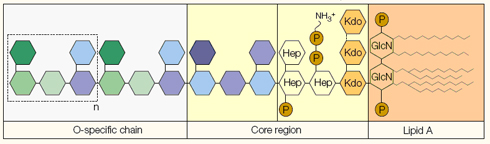
Figure 1. Structure of lipopolysaccharide showing its three main components (Beutler and Rietschel, 2006 Nat rev)
LPS Receptors
LPS induces a wide range of responses in host cells upon interaction with appropriate recognition receptors. In mammalian cells, LPS is recognised by a complex of proteins, the components of which include LPS binding protein (LBP), CD14, TLR4 and MD-2.
LBP is a 60kDa acute-phase protein which binds to the lipid A component of LPS. It is produced in the liver (Schumann et al., 1990), and also in the lung where it has important patho-physiological actions, including a role in ARDS and Gram-negative pneumonia amongst others. In LPS signalling, its main function is to catalyse the transfer of LPS from the outer membrane on to CD14 (Schumann et al., 1990).
CD14 is an approximately 50kDa glycoprotein which is expressed on the membranes of cells of monocytic origin, and to a lesser extent on neutrophils as a glycerophosphatidylinositol (GPI)-anchored molecule (membrane CD14, mCD14). It also exists as a circulating, soluble protein (soluble CD14, sCD14). Although LPS can directly bind membrane CD14 (mCD14), its affinity for the receptor is significantly enhanced when it forms a complex with LBP (Fenton et al.,1998). In certain cell types such as endothelial cells where mCD14 is lacking, sCD14 serves as the signalling receptor for surface recognition of the LPS-LBP complex (Goldblum et al., 1994). As CD14 lacks an intracellular cytoplasmic domain and essentially no intrinsic signalling capabilities, it transduces LPS signals through Toll-like receptor 4, the first identified member of the Toll-like receptor family (Medzhitov et al., 1997).
Evidence for the role of TLR4 in LPS signal transduction came from studies in LPS-unresponsive C3H/HeJ mouse strains which naturally contain a point mutation in the cytoplasmic signalling domain of the TLR4 protein. This mutation consists of a substitution of a conserved proline with histidine and results in a defect in TLR4-mediated signalling and a consequent suppression of the immune response to LPS (Poltorak et al., 1998). The critical role of TLR4 in the recognition of LPS was further confirmed through the development of TLR4-deficient mice; it was found that these mice were also hypo-responsive to LPS(Hoshino et al., 1999).
In addition to TLR4, another molecule, MD-2, an 18-25kDa protein which associates with the extracellular portion of TLR4 in the Golgi, is required for responsiveness to LPS (Shimazu et al., 1999). Evidence for its role has come from studies which have shown that MD-2 knockout mice are hypo-responsive to LPS and are resistant to LPS-induced endotoxin shock (Nagai et al., 2002). A number of other membrane receptors such as scavenger receptor (Kobayashi et al, 2000), TREM-1 (Nathan et al., 2001) and moesin (Tohme et al., 1999) are involved in the recognition of LPS.
References
Beutler B, Rietschel E.T. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3:169-176.
Dunn, D.L. 1991. Role of endotoxin and host cytokines in septic shock. Chest 100:164S-168S.
Fenton, M.J. and Golenbock, D.T. 1998. LPS-binding proteins and receptors. J Leukoc Biol 64:25-32.
Goldblum, S.E., Brann, T.W., Ding, X., Pugin, J., and Tobias, P.S. 1994. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J Clin Invest 93:692-702.
Hoshino, K., Takeuchi, O., Kawai, T., Sanjo, H., Ogawa, T., Takeda, Y., Takeda, K., and Akira, S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol 162:3749-3752.
Kobayashi, Y., Miyaji, C., Watanabe, H., Umezu, H., Hasegawa, G., Abo, T., Arakawa, M., Kamata, N., Suzuki, H., Kodama, T., et al. 2000. Role of macrophage scavenger receptor in endotoxin shock. J Pathol 192:263-272.
Medzhitov, R., Preston-Hurlburt, P., and Janeway, C.A., Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397.
Nagai, Y., Akashi, S., Nagafuku, M., Ogata, M., Iwakura, Y., Akira, S., Kitamura, T., Kosugi, A., Kimoto, M., and Miyake, K. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3:667-672.
Nathan, C. and Ding, A. 2001. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat Med 7:530-532.
Poltorak, A., He, X., Smirnova, I., Liu, M.Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088.
Schumann, R.R., Leong, S.R., Flaggs, G.W., Gray, P.W., Wright, S.D., Mathison, J.C., Tobias, P.S., and Ulevitch, R.J. 1990. Structure and function of lipopolysaccharide binding protein. Science 249:1429-1431.
Shimazu, R., Akashi, S., Ogata, H., Nagai, Y., Fukudome, K., Miyake, K., and Kimoto, M. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189:1777-1782.
Takada, H. and Kotani, S. 1989. Structural requirements of lipid A for endotoxicity and other biological activities. Crit Rev Microbiol 16:477-523.
Tohme, Z.N., Amar, S., and Van Dyke, T.E. 1999. Moesin functions as a lipopolysaccharide receptor on human monocytes. Infect Immun 67:3215-3220.