The differentiation of cells in three-dimensional constructs in-vitro
The stem cell phenotype may be defined as cells possessing proliferative potential along with capacity for self-renewal and the ability to differentiate into various lineages. Forming a minority population in a specific tissue, stem cells are maintained in a quiescent state of cell cycle with immediate responses to the signals transmitted by the niche (microenvironment). Pluripotent embryonic stem cells from the inner cellular mass (ICM) possess a wide range of differentiation capabilities both in-vitro as well as in-vivo (Banas et al., 2006). Pluripotent stem cells help provide insights to the elementary mechanisms and processes that determine the outcome of cells during the early stages of embryonal development (Przyborski et al., 2004). Pluripotent stem cells double up as basic tools for research in-vivo and vitro, as well as reliable material for testing a range of therapeutic products (Przyborski et al., 2004). Stem cells undergo spontaneous or induced differentiation both in 2D as well as 3D cell cultures (Gerecht-Nir and Itskovitz-Eldor, 2006).
The in-vivo environment is a complex architecture which maintains and modifies the cells as per the organism’s requirement. Cell to cell interactions are present within tissue along with extensive communications between cells and ECM (extra cellular matrix) (Bokhari et al., 2007b). These cell–cell interactions are executed via superficial cellular receptors which also facilitate interactions with ECM and numerous stimuli received from the microenvironment (Bokhari et al., 2007b). Early attempts for understanding the physiology and molecular mechanisms behind the cell differentiation and growth were mostly in-vivo (involving extensive use of animals) or based on experiments where cells were grown in Petri dishes (Bokhari et al.,2007b). There are numerous rules and regulations to be followed religiously when considering the usage of animals in laboratories and many organisations like P.E.T.A. are making a significant effort to reduce the usage of animals in such experiments (Sun et al., 2006). Numerous novel cell-based methods have been developed especially in the area of pharmaceutical research (Taylor et al., 2001). The newly adopted cell-based methods mimicked the in-vivo cell behaviour and thus helped to achieve results with reduced cost, improved efficiency and helped predict accurate drug behaviour (Bhadriraju and Chen, 2002). For many decades the 2D environment with its low cost and recognized efficiency had been the gold standard for all cell-based studies, especially for drug discovery and testing (Giese et al., 2002). But with new insights and discoveries, various limitations of the 2D microenvironment became evident such as changes in cell shape; ECM production, mitosis and gene expression are brought about by cell-microenvironment interaction (Gruber and Hanley Jr., 2000).
Experiments involving 2D cell growth failed to mimic the architecture and microenvironment that the cell experiences in-vivo (Sun et al., 2006) as a result of which, many times, ambiguous or unsatisfactory data was obtained (Weaver et al., 1997) (Sun et al., 2006). Mostly only one cell type is taken into account in a 2D environment and the impact that the surrounding cells and tissues have on the growth of other cells is never considered (Birgersdotter et al., 2005; Sun et al., 2006). Hence a change of approach introduced 3D environments for cell growth in-vitro. A 3D environment mimics most of the geometric and mechanical aspects of an in-vivo environment due to which the cells remain surrounded by ECM and other cells in the vicinity (Sun et al., 2006). It has been experimentally proven that ECM in a 3D environment promotes normal epithelial differentiation and growth (Ingber et al., 1995). Experimentally proven facts also include the formation of more rounded phenotype of cells, a rise in proteoglycan synthesis and the formation of multi-celled colonies with ECM deposits in and around the cells (Gruber and Hanley Jr., 2000). Cells grown on a monolayer exhibit a flat and spindle-shaped phenotype (Gruber and Hanley Jr., 2000) which totally contradicts with the morphology of the cells observed in-vivo. As a result of which, laboratories are trying to culture cells in a 3D environment and formulate novel approaches for maximizing results and minimizing errors. Culturing any type of cell in Petri dishes and encouraging them to adopt morphologically similar phenotypes of the desired cell type is challenging. Knowledge gained from cell differentiation and proliferation has led to many discoveries. Many technologies have been tested for supporting the growth of cell in a 3D environment. Several other unique approaches have been generated for 3D growth of cells. Development of biodegradable polymers like poly (glycolic acid), poly (lactic acid) (Mikos et al., 1993), use of hydrogels containing mixture of agarose, collagen and/or fibrin (Blackshaw et al., 1997). Biodegradable scaffolds are considered to be a prerequisite for transplantations of the future and hydrogels provide an excellent microenvironment for 3D growth of cells. The hanging drop is also another ancient technique practiced in today’s modern tissue culture labs. It involves setting up drops of cells on the lid of the Petri dish and inverting it on the dish itself which is filled with PBS (phosphate buffered saline) for humidifying the chamber (Gutiérrez et al., 2005)(Banerjee and Bhonde, 2006). The main advantages of this system are the cost, the total number of cells and quantity of medium used are minimized (Gutiérrez et al., 2005)along with maintenance of wide variety of cell lines (Banerjee and Bhonde, 2006).
Another approach is the development of 3D scaffolds polymerized in high internal phase emulsions (HIPEs) (Bokhari et al., 2007a), a novel technology developed by ReInnervate Ltd (http://www.reinnervate.com/). The polystyrene scaffold fabrication consists of a cascade of steps starting from the removal of inhibitors from monomers, to preparation of monomer phase, formation of HIPE and its polymerization ultimately forming POLYHIPE (Polymerized High Internal Phase Emulsions). The main advantages of using this system lies in the ability to control the morphology and material properties of the resulting foam, which can be of great use for culturing different types of cells where it can be altered accordingly (Carnachan, et al., 2006).
However, there are pitfalls to these methods, the possibility of different types of cells reacting differently to a degrading scaffold has not been studied extensively, practical applications of hydrogel are stunted by factors like gel penetration, cost and storage. The volume of the liquid used in a hanging drop culture is less than or equal to 50 μl for maintaining the drop on the lid via surface tension, because of which it is also extremely difficult to observe any form of growth in the drop by microscopy. In this type of assay, it is impossible to change the media so the cells can only be cultured for a short time. Most of the novel techniques are developed with core objectives like tissue engineering and transplanting the in-vivo grown tissue into a live host for regeneration of the organ. Less attention has been paid for the development of a 3D cell culture model specifically for the laboratory. Hence a scaffold that is inert, non-degradable and supports the growth of cells is preferred for routine use. Also the cells grown on a polystyrene scaffold respond to a variety of biochemical agents in a similar fashion to the cells growing in-vivo (Bokhari et al., 2007b).
Comparative study between 2D and 3D cell growth
Cells used for this experiment were derived from a single stock of cells to minimize errors. TERA2 SP12 cells were cultured in a 12 well plate coated with Matrigel to mimic the 2D environment. Matrigel was thawed overnight in ice kept at 4°C and coated onto the 12 well plates on the day of the experiment. The coated plates were incubated at 37°C for 30 minutes before loading the cells. About 100,000cells/ml were loaded in the 12 well plates and incubated at 37°C in 5% CO2. Well inserts were prepared for the 6 well plates and about 100,000cells/ml, 500,000cells/ml and 1 million cells/ ml were loaded on the inserts in the 6 well plates, to which about 4 ml of media was added and incubated at 37°C in 5% CO2. A total of 8 plates were produced (4 containing Matrigel and 4 with without Matrigel) to measure and observe the growth and differentiation pattern over time intervals of 4, 7, 14 and 21 days. Retinoic acid was added to the media in all plates to induce cell differentiation and study the effect of the combination of Matrigel and retinoic acid on stem cell development.
Results
Comparative study between 2D and 3D cell growth
2D Results
There were significant differences observed between the cells grown on 2D and 3D environments. The EC stem cells loaded in the 12 well plates (+/- Matrigel) were processed over time intervals of 4, 7, 14 and 21 days. EC cells gown on 2D plastic plates for 4 and 7 days appeared as flat structures. The cells were not confluent and had a disorganised appearance. Few isolated dead cells were also observed after 14 to 21 days; the cells were clustered together but did not exhibit any 3D structures resembling the ones observed in the stained teratoma slides and also in the 3D scaffolds. The presence of Matrigel had a positive impact on the cell proliferation in the 2D environment. Immunoflourescence staining of cells grown in 2D cell culture was also carried out using TUJ 1 (Mouse IgG; expressed in neurons, red fluorescence) and NF 200 (mature neuronal marker, green fluorescence). There was a linear increase in the number of mature neuronal cells as exhibited by staining with NF 200; however, the number of cells expressing TUJ 1 was lower compared to the 21st day cell culture of 3D scaffolds.
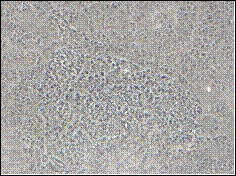
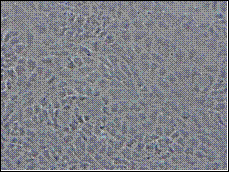
A (21 days) B (14 days)
Fig. 1: Phase contrast microscopy showing growth of TERA 2.cl.SP 12 EC cells cultured in the presence of retinoic acid (RA). A) TERA 2 EC cells cultured in 12 well plates after exposure to retinoic acid for 3 weeks exhibited better differentiation and confluence as compared to other plates; B) TERA 2 cells cultured in 12 well plates after exposure to retinoic acid for 2 weeks appeared as flat cells without any complex structures. Scale: A) 100 X; B) 200 X.
3D results
The cells on the 3D scaffolds were also grown and processed over a time interval of 4, 7, 14 and 21 days; they exhibited an exponential growth pattern with slight anomalies observed in the cells grown and processed after 14 days. There was also a significant difference observed between the number of cells grown with and without Matrigel. In almost all of the scaffolds, TERA cells formed spherical-shaped structures which were actually rounded clusters of closely packed cells; also confirmed by images taken by the electron microscope in a separate experiment. In the scaffolds processed after 7 days there was a clear difference observed in the number of cells supplied with Matrigel and ones grown without Matrigel. The TERA 2 cells grown with Matrigel have almost filled up the scaffold as compared to the cells grown without the Matrigel. A lot of spherical structures were also being observed in the scaffolds with Matrigel and the cells appeared to occupy the cup-shaped interconnecting 3D voids. The scaffolds processed after 14 days exhibited less cell growth compared with the 7 and 21 days, the reason for this anomaly in unknown. In spite of this, more mature and lineage-oriented cells were observed, mainly because of the addition of retinoic acid into the plates. Early stages of gland like formations were observed in the 14- as well as 21-day scaffolds which were very similar to ones observed in trichrome-stained teratoma samples, which were supplied by Re-innervate Ltd. (pre-sectioned and ready for staining).
The scaffolds processed after 21 days had obviously the densest population of cells, covering almost the entire scaffold with less room left for expansion. A lot of close cell–cell interaction was observed with a limited number of dead cells. Increase in the number of spherical structures and the early gland-like formations were also observed. Also there was less evident difference observed between the numbers of cells cultured with and without Matrigel; however, the presence of Matrigel did affect the morphology since the cells cultured in its absence appeared to be unhealthy and disintegrating. It has been documented that retinoic acid induces the stem cells towards neuronal lineages in a 2D environment (Stewart et al., 2003). Immunoflourescence staining of 7- and 21-day scaffolds revealed that cells grown on the 3D scaffolds were also maturing towards neuronal lineages. TUJ 1 (Mouse IgG; expressed in neurons, red fluorescence) and GFAP (marker of astrocytes, green florescence) were the two primary antibodies used for immunoflourescence staining of the scaffolds processed after a week while TUJ 1 and NF 200 (mature neuronal marker, green fluorescence) were used on the 21-day ones. The 7-day scaffolds had less fluorescence indicating that only few of the cells were lineage specific. The 21-day scaffolds on the other hand had a large population of mature lineage-specific cells. In almost all the scaffolds the initial number of cells which were loaded had a huge impact on the growth and differentiation of cells. The scaffolds loaded with 100,000 TERA 2 cells had less cell proliferation compared to 500,000 and 1 million cells with the presence of large numbers of dead cells.
Masson’s Trichrome-stained 3D scaffolds:
7th day
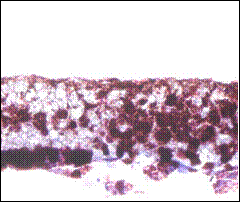

A B
21st day
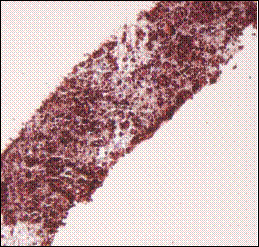
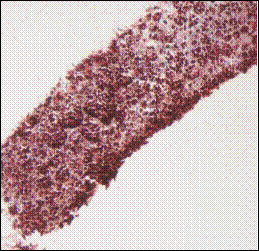
Fig. 2: Masson’s Trichrome staining of 1 million TERA 2.cl.SP 12 stem cells after culturing and processing the scaffold after one and three weeks in the presence of retinoic acid. A) TERA 2 cells cultured on the 3D scaffold in the absence of Matrigel; B) Same line and number of cells loaded on the scaffold but in the presence of Matrigel; C) 1 million cells processed after 3 weeks cultured on 3D scaffold without Matrigel; D) Same number of cells cultured in same conditions but in the presence of Matrigel. Clearly the presence of Matrigel is enhancing the growth and proliferation of TERA 2 cells cultured on the 3D scaffold. Scale: A) 20X, B) 20X, C) 20X, D) 20X.
Comparison of complex structures 3D scaffold:
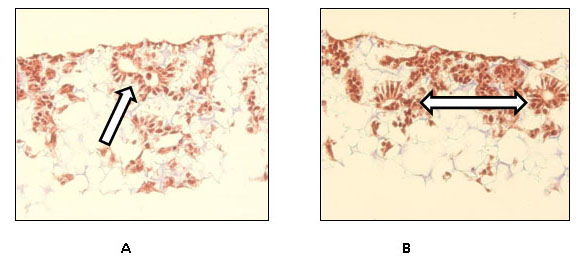
14th day
21st day
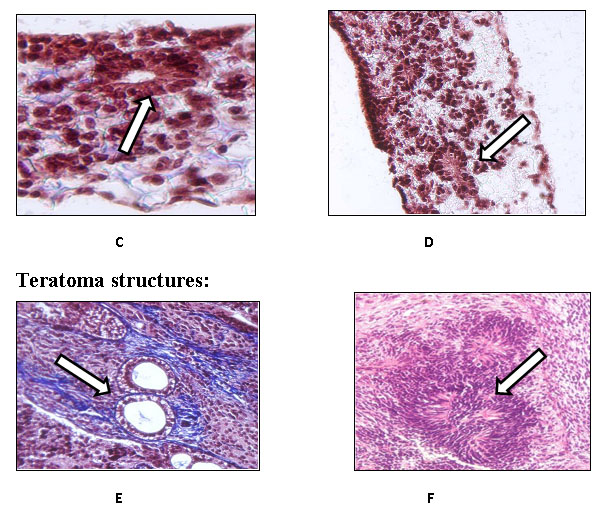
Fig. 3: Masson’s Trichrome staining of the complex structures formed by TERA 2.cl.SP 12 loaded and cultured on the scaffold in the presence of retinoic acid (A-D) and the complex structures visible in a teratoma (E, F). These are the examples of TERA 2 cells cultured on 3D scaffolds in the presence of Matrigel and processed after A) 14 days with the arrow pointing towards a visible complex structure; B) 14 days as well with the double arrow pointing towards the two visible complex structures; C) after 21 days with the arrow pointing towards a gland-like structure and D) also after 21 days with the arrow pointing at a possible neural rosette. The remaining two are the examples of stained complex structures found in teratomas. E) Trichrome stained complex structure with an arrow pointing towards a gland like structure and F) the arrow pointing towards a neural rosette observed in a teratoma. Clearly some morphological similarities do exists between the structure observed in 3D scaffolds and the ones observed in teratomas concreting the fact that cell when cultured in a 3D environment form more complex structures similar to ones present in-vivo.
Conclusion
This experiment proves that a 3D environment is better suited for cell cultures hence all ambiguous data obtained from the 2D cultures can be ruled out by using this kind of cell culture apparatus for cell expansion. The cells form complex structures which closely resemble their in-vivo counterparts so they could lead to an animal-free approach for drug and toxicological testing. However, to reach this stage, a comparative study (i.e. immunoflourescence staining) between the structures observed on the scaffold and in-vivo have to be carried out. The presence of retinoic acid did stimulate the cells towards neuronal lineage in 3D scaffolds and the presence of Matrigel not only enhanced cell growth but also helped in enhancing the morphology of cells. TERA2.cl.SP12 human embryonal carcinoma cells were used for these experiments which are a robust line of cells, the future experiments will deal with growth and proliferation of human embryonal stem cells (ES cells) or murine stem cells and using EC 23 and BMP instead of retinoic acid as an external stimuli. Therefore, this system of cell culture has proved very efficient for culturing EC stem cells; however, a lot of research is still required in the field of 3D scaffolds for culturing different types of cells with the ultimate goal of transplantation for regenerating damaged organs.