Importance of NR2A and NR2B subunit NMDARs in LTP and LTD
Introduction
Acting as a glutamate-gated ion channels N-methyl D-aspartate receptors (NMDARs) play an important role in excitatory synaptic transmission. They are widely expressed in the central nervous system and have a key role in synaptic plasticity. NMDAR channels, if blocked, result in the inhibition of cellular mechanisms like long-term potentiation (LTP) and long-term depression (LTD) that are important for learning and formation of memory.
An NMDA channel’s subunit composition defines its biophysical, pharmacological and molecular properties such as the role of divalent cations, kinetic properties and interaction with intracellular proteins (Cull-Candy et al., 2001). It is proposed that NMDA receptors are composed of four subunits of which NR1 subunits occupy two while the remaining two could be made up of NR2A-D or NR3A-B regulatory subunits. NR1 has eight splice variants and apart from NR2A all other subunits of NR2 family have splice variants, individual functions of which are not yet known. However, this essay specifically focuses on subunit-specific NR2A and NR2B channels as they are the most studied form of NMDARs. The expression of NR2A channels is observed all over the brain at nearly three weeks postnatal, while there is no expression seen on postnatal day (P) 0. On the other hand, NR2B that is ubiquitously found at birth is found to be restricted to the forebrain in adults (Monyer et al., 1994). It is observed that the expression of both these receptors is developmentally regulated with varying relative expression that has been shown to be important in long-term synaptic plasticity (Foster et al., 2010).
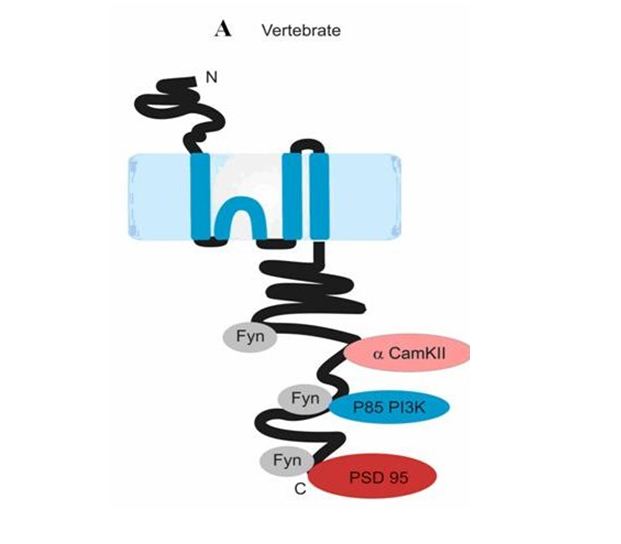
A lot of studies in the last decade have hypothesized about the different roles of NR2A and NR2B receptors in hippocampus in the managing of induction of synaptic plasticity. However, a precise understanding of the molecular mechanisms that these NMDA receptor complexes are involved in is still missing. NR2A channels as compared to NR2B channels have faster rise and decay kinetics, but NR2B channels carry nearly two-fold more charge in a single activation when compared to NR2A channels (Yashiro and Philpot, 2008), although more studies are required to confirm this. Both kinds of receptors are found synaptically and at extrasynaptic locations. However, their role based on their location is debatable. The interactions of these channels with intracellular proteins define their role in synaptic plasticity to a certain extent, such as binding of NR2B subunit with CaMKII (Yashiro and Philpot, 2008) and that of both NR2A and NR2B to PSD-95, a protein of the MAGUK (membrane-associated guanylate kinase) family.
Depending on the developmental stage, the ratio of NR2A to NR2B varies which is suggested to play a part in the changing role of individual channels in LTP and LTD. As mentioned above, NR2A channel expression increases with development resulting in increasing NR2A/NR2B ratio, which alters the synaptic plasticity threshold as observed by Philpot et al. (2007).
There has been significant evidence that supports the essential role of NR2B in the induction of LTP. Conversely, there also have been studies showing the importance of NR2A in LTP that have been questioned because of the use of antagonist NVP-AAM077 (NVP) that was reported to have 100-fold specificity for NR2A over NR2B-cloned human NMDARs in exogenous cell lines (Liu et al., 2004), but studies have found it to be only 6-12 fold specific for NR2A in heterologously expressed rodent NMDARs (Yashiro and Philpot, 2008).
This essay focuses on a study undertaken by Bartlett et al. (2007) that utilizes NVP and Ro-25-6981 (Ro) antagonists to show their specific role in altering LTP and LTD by acting on NR2A and NR2B recombinant rat receptors in HEK293 cell lines. Ro is highly selective for NR2B receptors. In the same direction as the Bartlett et al. paper, Gardoni et al. (2009) in the second paper confirm the importance of NR2B in LTP by the inhibition of Calmodulin-dependent Protein Kinase II (CaMKII) for a brief period of time. However, the third paper reveals the importance of the presence of cytoplasmic tails of NR2A and NR2B receptors in LTP.
Results obtained by chosen papers
Studying the Bartlett et al. paper gives us clues of importance of both NR2A and NR2B receptors in LTP at P14 in CA1 synapses at a time when expression of both receptors can be seen. Also, their results tell us about the NR2B receptor’s role in LTD. In accordance with that, when low concentration (0.1μM) of NVP was used, only NR2A receptors were blocked with 63% blockage of LTP, while, on higher concentrations (0.4μM), nearly 96% of LTP was blocked. It was also observed that NVP at these concentrations (0.4μM) blocked NR2B receptors to some extent. Also, Ro blocked LTP by around 45% with a partial block of NR2B receptors (60%) at concentrations between 3-5μM. There was a complete block of LTP observed when both NVP and Ro were used at the same time, suggesting NVP also blocks NR2B to a certain extent. LTD was not inhibited by a block of NR2B (partial block by Ro), whereas, NVP at higher concentrations (0.4μM) inhibited LTD as well. The authors hint at the role of other NR2 (NR2C and NR2D) subunits in the inhibition of LTD by mentioning the possible role of NVP in their blockage as stated by previous studies (Feng et al., 2004). Based on the results of their study, they could not predict a role of NR2B in LTD.
Following the suggested importance of NR2B in LTP from findings of Bartlett et al., results of work from Gardoni et al. led to an understanding of a molecular mechanism that is involved in the induction of LTP through NR2B receptors. Taking cues from their previous work on the interaction of cytoplasmic tail of NR2A/B receptors with CaMKII and PSD-95 (Gardoni et al., 1998) and the work of Barria and Malinow (2005) about the role of the NMDA-CaMKII complex in synaptic plasticity, the authors in this paper show that the inhibition of CaMKII in 14 days in vitro (DIV) rat hippocampal culture neurons from embryonic day E18-19 for a brief period of two hours results in redistribution of NR2B receptors between synaptic and extrasynaptic locations, which affects the induction of LTP in the correct manner. They obtain the same result by disrupting the NR2B/PSD-95 complex using TAT-2B where NR2B expression was not altered. The role of CaMKII in synaptic plasticity has been previously described (Colbran and Brown, 2004) and, in this study, Gardoni et al. used this knowledge by inhibiting CaMKII using Ant-AIP-2 (antennapedia peptide (Ant) fused with autoinhibitory peptide) that resulted in the inhibition of CaMKII that also resulted in an approximately 50% decrease of NR2B subunit receptor from synaptic regions, this was observed using confocal imaging. However, overall the concentration of NR2B receptors was found to be nearly the same. The lateral movement of NR2B receptors had taken place as concluded from the observation that NR2B intracellular content had not changed when chymotrypsin was used. So in order to measure the surface expression, the concentration of phosphorylated CREB was studied because a previous study demonstrated that extrasynaptic NR2B receptors trigger dephosphorylation of CREB, while synaptic ones promote phosphorylation of the same (Hardingham et al., 2002). It was seen that CaMKII inhibition lowered the phosphorylated CREB, but the overall amount of CREB remained the same. Furthermore, studies using immunoprecipitation techniques showed that NR2B/αCaMKII complex was disrupted due to Ant-AIP-2, resulting in their reduction at synapses. Using field recording experiments on CaMKII inhibited hippocampal slices, it was seen that LTD was not affected while LTP induction was severely impaired and High Frequency Stimulations (HFS) did not result in LTP as they otherwise should have, also, TAT-2B, which also results in inhibition of LTP by disrupting NR2B/αCaMKII complex, did not have any effect on LTD.
Foster et al. show us the importance of cytoplasmic tails of NR2A and NR2B receptors in the induction of LTP by RNA interference (RNAi) methods on organotypic hippocampal slice cultures. They use pharmacological approaches to confer results, the same as previous studies that showed that NR2A concentration increases with time which results in independence from NR2B. However, when NR2B expression is blocked using RNAi, LTP induction does not take place. Clayton et al. (2002) reached a similar conclusion when antisense oligonucleotides were used to inhibit the expression of NR2B. This abnormality can be rescued by the overexpression of RNAi-resistant NR2B. On the other hand, they suggest that NR2A expression is not essential for LTP and NR2A’s cytoplasmic tail seems to inhibit LTP.
Experiments from the Foster et al. study confirm that Ro completely blocked LTP at DIV 6-8, whereas, at around DIV 11-14, NR2B blockage by Ro did not affect the induction of LTP. Thus, LTP no longer required activation of NR2B channels. NMDA-EPSC (excitatory postsynaptic currents) contribution from NR2B drops from 74% to 64% at this stage, which further goes down to approximately 38% by DIV 18-21. In support of their previous work that NR2B NMDARs are required for supporting NR2A expression (Kim et al., 2005), Foster et al. showed that NR2B-RNAi resulted in the loss of NMDA-EPSC amplitude but not of half-width of NMDA-EPSC which was only slightly altered. This also resulted in a 1.7-fold decreased LTP in the transfected cells compared to untransfected ones. Using silent mutations at the target site of RNAi, RNAi-resistant NR2B cDNA (NR2B*) was prepared that rescued LTP, also resulting in slightly increased half-width of NMDA-EPSC. Contrary to previous results, when NR2A-RNAi was used, compared to untransfected cells, the LTP magnitude was a little higher despite the mean NMDA-EPSC amplitude going down. Coexpression of NR2A in this case did not rescue LTP, also, when NR2A was used alone on transfected cells, LTP showed diminished display. All of this when interpreted together showed that NR2B was essential for LTP while NR2A was not at DIV 11-14 slice cultures.
Using the cytoplasmic tail of NR2B, which represents nearly half of the protein, chimera NR2A-2Btail and NR2B*-2Atail were used to find out the importance of physical presence of NR2B. It was observed that NR2B*-2Atail failed to restore LTP while increasing NMDA-EPSC amplitude to approximately 65% from 25%. On the contrary, NR2A*-2Btail restored LTP. Thus, the resulting observation suggests the importance of an NR2B cytoplasmic tail for LTP. Also, either of the NR2A or NR2B channel can support LTP when fused with an NR2Btail. LTP was not rescued if the NR2Btail was altered. But, surprisingly, the altered tail of NR2A was able to rescue LTP, which suggests the presence of an LTP inhibitor in the NR2Atail may recruit proteins that inhibit LTP.
Discussion
Bartlett et al.’s study suggests about the important role of both NR2A and NR2B in LTP induction in two-week-old rats cannot account for their role in LTD, as NVP’s role in the inhibition of NR2 receptors is still not clear. NR2B is not completely inhibited by Ro and there are marked changes in receptor concentration with development. As mentioned before, taking into consideration the importance of NR2B in LTP from previous papers, Gardoni et al. in their paper show us the importance of interaction between a NR2B cytoplasmic tail and CaMKII for the induction of LTP; disrupting the NR2B/αCaMKII complex by inhibition resulted in the loss of LTP induction along with loss of NR2B receptors from synaptic locations. However, none of this affected LTD induction in any manner. On the other hand, contrary to their results that movement of NR2B takes place laterally and not intracellularly, a recent study by Gambrill et al. (2011) suggests that movement of NMDARs may be lateral or intracellular until equilibrium is reached under such conditions. Their results regarding the role of NR2B c-terminal peptides go with the previous study by Aarts et al. (2002). Bringing into consideration the work of Foster et al., the importance of a cytoplasmic tail of both NR2A and NR2B in LTP induction is clearly presented. They bring together pharmacological and molecular approaches to reach the conclusion that there is a collaboration of both NR2A and NR2B receptors where NR2A takes the role of a conducting channel while the NR2B tail recruits proteins for LTP. Also, the important role of the NR2A tail as a recruiting inhibitory factor of LTP induction was seen, which was altered because of mutation, which resulted in the induction of LTP even in the presence of RNAi of NR2B receptors. An important question that arose from this observation was how was the mutant NR2A tail able to rescue LTP? NR2B-RNAi was not able to inhibit all of NR2B, the remainder of which helped the NR2Amutant-tail to rescue LTP altogether.
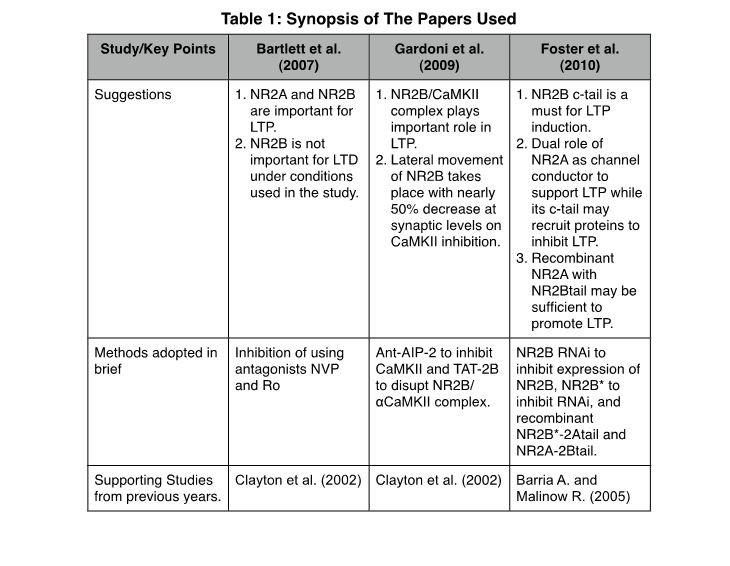
All of the studies leave us with no doubt about the importance of NR2A and NR2B receptors in LTP, and the role of both of them, as a conducting channel and as a protein recruiter needs to clearly defined by further intensive studies. These papers clearly present a timeline in the steady development of our understanding of the molecular and as well as pharmacological mechanisms involved in the functioning of NMDARs with respect to synaptic plasticity (Table 1).
References
Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M (2002) Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298:846 – 850.
Barria A, Malinow R (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48:289–301.
Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D (2007) Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacol- ogy 52:60 –70.
Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD (2002) A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci 22:3628 –3637.
Colbran RJ, Brown AM (2004) Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 14: 318-327.
Cull-Candy S, Brickley S, Farrant M (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335.
Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT (2004) Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141: 508-516.
Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M (2010) Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci 30:2676–2685.
Gambrill AC, Storey GP, Barria A (2011) Dynamic Regulation of NMDA Receptor Transmission. J Neurophysiol 105:162-171
Gardoni F, Mauceri D, Malinverno M, Polli F, Costa C, Tozzi A, Siliquini S, Picconi B, Cattabeni F, Calabresi P, Di Luca M (2009) Decreased NR2B subunit synaptic levels cause impaired long-term potentiation but not long-term depression. J Neurosci 29:669–677.
Gardoni, F., Caputi, A., Cimino, M., Pastorino, L., Cattabeni, F. & Di Luca, M. (1998) NR2A/B subunits of NMDA receptor as anchors for CaMKII in Post Synaptic Densities. J. Neurochem., 71, 1733–1741.
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neuroscience 5: 405-414.
Kim MJ, Dunah AW, Wang YT, Sheng M (2005) Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 46:745–760.
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:1021–1024.
Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529 –540.
Philpot BD, Cho KK, Bear MF (2007) Obligatory Role of NR2A for Metaplasticity in Visual Cortex. Neuron 53:495–502.
Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55:1081–1094.
Cover Image: Model of NR2 subunit. Ryan et al. (2008) Evolution of NMDA receptor cytoplasmic interaction domains: implications for organisation of synaptic signalling complexes. BMC Neurosc. 9:6